The CHADS2 score is a proven, essential tool for estimating cardioembolic risk (mainly stroke) in patients with nonvalvular atrial fibrillation, with the purpose of determining the indication for anticoagulant therapy. In this study we analyzed the use of CHADS2 in hypertensive patients without known atrial fibrillation in a Mediterranean population.
MethodsThe study included 887 hypertensive patients aged 65 years or older without atrial fibrillation or anticoagulant therapy, who attended a medical consultation. Data on the patients’ main risk factors, cardiovascular history, and medication were collected, basic laboratory analyses and electrocardiography were performed, and the CHADS2 score (heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and previous stroke or transient ischemic attack) was calculated. A clinical follow-up was carried out, recording hospital admissions for a stroke or transient ischemic attack. The median duration of follow-up was 804 days.
ResultsMean age was 72.5 (SD,5.7) years, 46.6% were men, 27.8% had diabetes, and 8.6% were smokers. During follow-up, 40 patients were hospitalized for a stroke or transient ischemic attack (4.5%). The event-free survival analysis showed significant differences according to the CHADS2 score (log rank test, P < .001). On multivariate analysis, smoking and CHADS2 ≥3 were independent predictors of stroke or transient ischemic attack.
ConclusionsThe CHADS2 may be useful for estimating the risk of stroke or transient ischemic attack in hypertensive patients without known atrial fibrillation.
Keywords
The CHADS2 score is a clinical predictor of the risk of stroke in patients with nonvalvular atrial fibrillation, used to determine whether anticoagulant or antiplatelet therapy is indicated.1 It is a simple rule that is easy to remember and apply in clinical practice, and it has been validated in several studies.2,3 This has facilitated widespread adoption of CHADS2 and support for its use among major scientific societies in Spain and elsewhere.4–6 The current European guidelines have incorporated additional stroke risk factors in the score to improve identification of patients “at low risk” (CHA2DS2-VASc).7
Despite the proven utility of the CHADS2 score and other risk stratification approaches in patients with nonvalvular atrial fibrillation, most ischemic strokes (85%) occur in individuals without known atrial fibrillation.8 Moreover, epidemiologic studies have shown that hypertension is the most important determinant of stroke risk, and that each component of the CHADS2 score is independently associated with cerebrovascular events in the general population.9 Nonetheless, to our knowledge, there are no studies investigating the utility of this score for estimating the risk of a cerebrovascular event in hypertensive patients without known atrial fibrillation. The aim of this study was to analyze the role of the CHADS2 score to estimate stroke risk in a sample of hypertensive patients aged 65 years or older and in sinus rhythm who attended several centers in a Mediterranean area.
METHODSThe FAPRES registry is an observational, multicenter, epidemiologic study performed in the clinical care setting and designed to acquire information on the prevalence of atrial fibrillation in patients aged 65 years and older with a clinical diagnosis of hypertension, living in the Valencian Community of Spain. Sixty-nine investigators from primary care centers and hospital hypertension units in Alicante, Castellón, and Valencia participated in the study, in percentages consonant with the population density of each of the 3 provinces. A detailed description of the study and definition of the variables has been reported previously.10 A total of 1028 patients were included in the baseline study. The investigators were invited to carry out a 2-year clinical follow-up of these patients, compiling information on the main cardiovascular events. All patients gave informed, written consent for participation, and the study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee for Clinical Research of Hospital General Universitario de Castellón.
Study PopulationThe study included all patients recorded in the FAPRES registry who showed sinus rhythm on the baseline echocardiography, were not receiving anticoagulant therapy, and whose medical records showed no history of atrial fibrillation. The patients’ cardiovascular history and risk factors were recorded on a standardized questionnaire. Patients who used some type of tobacco (cigarettes, pipe tobacco, cigars, or smokeless tobacco) in at least the previous month were considered smokers,11 whereas those who had stopped smoking at least 1 year previously were considered former smokers. Patients who actively walked at least 30minutes per day or engaged in some type of sport at least 3 days per week were considered to practice physical exercise.12 We recorded the drug therapy being taken by patients at the time of the medical visit, specifically antihypertensive agents and preventive treatment for cardioembolic stroke (anticoagulant and antiplatelet drugs).
Anthropometric data (weight, height, and waist perimeter) and blood pressure values were recorded at the physical examination. Blood pressure measurement adhered to recommendations from clinical practice guidelines13: blood pressure was measured using calibrated, automated devices with the patient seated and following a 5-minute rest on 2 separate occasions, 2minutes apart. The mean of the 2 values obtained was then calculated. Analytical information was requested from the attending laboratory or obtained from the patients’ medical records when data from the previous 6 months were available. The glomerular filtration rate was calculated using the MDRD (Modification of Diet in Renal Disease Study) formula. The clinical history questionnaire was sent to a CRO (contract research organization) for automatic data processing. The results of electrocardiography study, performed in all patients, were sent by ordinary mail to a reference center, where they were independently analyzed by 2 experienced cardiologists blinded to the patients’ clinical data. The readers evaluated the presence of atrial fibrillation and left ventricular hypertrophy using the Sokolov criteria, Cornell criteria, or ventricular overload. A randomized external audit of 10% of the questionnaires was done to verify the reliability of the data included.
The CHADS2 score was determined in all patients to assess stroke risk (congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus, 1 point each, and prior stroke or transient ischemic attack [TIA], 2 points),2 and the patients were divided into 4 groups according to the score: 1, 2, 3, or ≥ 4 points. The patients underwent clinical follow-up with recording of hospitalization for stroke or TIA.
Statistical AnalysisThe data compiled in the study are expressed in terms of the central tendency, measures of dispersion, and relative frequencies. Quantitative variables were compared between groups with the Student t test or ANOVA, and categorical variables with the chi-square test. Event-free survival (stroke/TIA) according to the CHADS2 score was calculated with the Kaplan-Meier method. Multivariate logistic regression analysis was used to determine variables independently related to the incidence of cerebrovascular events during follow-up. Logistic regression included all the variables that were significant on univariate analysis, variables with recognized clinical significance, and the CHADS2 score. A receiver operating characteristic (ROC) curve was constructed and the area under the curve was calculated to analyze the validity of the CHADS2 score for estimating the risk of stroke/TIA. Furthermore, a combined variable including CHADS2 and significant variables on multivariate analysis was created, and again, the ROC curve was calculated to predict the risk of stroke/TIA. A P value of less than .05 was considered significant. SPSS version 21 was used for the statistical analyses.
RESULTSOf the 1028 hypertensive patients included in the baseline FAPRES study, we selected 922 patients without known atrial fibrillation who were not receiving anticoagulant therapy; 887 of them completed follow-up (96.2%) in a median of 804 (723-895) days. The mean age of the population was 72.5 (SD,5.7) years, and 46.6% were men. Relevant background included hypercholesterolemia in 47.8%, diabetes mellitus in 27.8%, and smoking in 8.6%. In addition, 62 patients (7%) had a previous stroke, 31 (3.5%) a diagnosis of heart failure, and 115 (13%) ischemic heart disease.
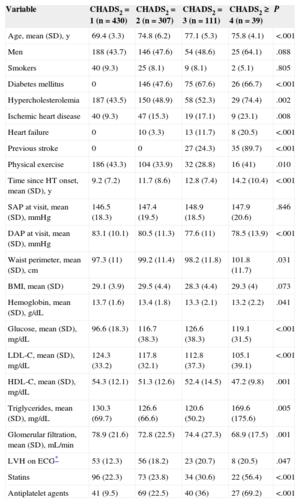
On calculation of the CHADS2 score, 430 patients (48.5%) were found to have a score of 1, 307 (34.6%) 2, 111 (12.5%) 3, and 39 (4.4%) ≥ 4. The main characteristics of the study population according to CHADS2 score are shown in Table 1. Patients with higher scores were older, had a greater prevalence of risk factors and established cardiovascular disease (in particular, ischemic heart disease and left ventricular hypertrophy), and had been hypertensive for longer than those with lower scores. Plasma high-density lipoprotein cholesterol concentrations and glomerular filtration rates were lowest in the group with the highest CHADS2 score. As to treatment, patients with CHADS2 ≥ 4 were taking angiotensin II receptor blockers, calcium channel blockers, statins, and antiplatelet therapy more frequently than the other patients. There were no differences in the use of angiotensin converting enzyme inhibitors, beta-blockers, or diuretics among the 4 groups.
Baseline Characteristics of the Population, Stratified According to CHADS2 Score
| Variable | CHADS2 = 1 (n = 430) | CHADS2 = 2 (n = 307) | CHADS2 = 3 (n = 111) | CHADS2 ≥ 4 (n = 39) | P |
|---|---|---|---|---|---|
| Age, mean (SD), y | 69.4 (3.3) | 74.8 (6.2) | 77.1 (5.3) | 75.8 (4.1) | <.001 |
| Men | 188 (43.7) | 146 (47.6) | 54 (48.6) | 25 (64.1) | .088 |
| Smokers | 40 (9.3) | 25 (8.1) | 9 (8.1) | 2 (5.1) | .805 |
| Diabetes mellitus | 0 | 146 (47.6) | 75 (67.6) | 26 (66.7) | <.001 |
| Hypercholesterolemia | 187 (43.5) | 150 (48.9) | 58 (52.3) | 29 (74.4) | .002 |
| Ischemic heart disease | 40 (9.3) | 47 (15.3) | 19 (17.1) | 9 (23.1) | .008 |
| Heart failure | 0 | 10 (3.3) | 13 (11.7) | 8 (20.5) | <.001 |
| Previous stroke | 0 | 0 | 27 (24.3) | 35 (89.7) | <.001 |
| Physical exercise | 186 (43.3) | 104 (33.9) | 32 (28.8) | 16 (41) | .010 |
| Time since HT onset, mean (SD), y | 9.2 (7.2) | 11.7 (8.6) | 12.8 (7.4) | 14.2 (10.4) | <.001 |
| SAP at visit, mean (SD), mmHg | 146.5 (18.3) | 147.4 (19.5) | 148.9 (18.5) | 147.9 (20.6) | .846 |
| DAP at visit, mean (SD), mmHg | 83.1 (10.1) | 80.5 (11.3) | 77.6 (11) | 78.5 (13.9) | <.001 |
| Waist perimeter, mean (SD), cm | 97.3 (11) | 99.2 (11.4) | 98.2 (11.8) | 101.8 (11.7) | .031 |
| BMI, mean (SD) | 29.1 (3.9) | 29.5 (4.4) | 28.3 (4.4) | 29.3 (4) | .073 |
| Hemoglobin, mean (SD), g/dL | 13.7 (1.6) | 13.4 (1.8) | 13.3 (2.1) | 13.2 (2.2) | .041 |
| Glucose, mean (SD), mg/dL | 96.6 (18.3) | 116.7 (38.3) | 126.6 (38.3) | 119.1 (31.5) | <.001 |
| LDL-C, mean (SD), mg/dL | 124.3 (33.2) | 117.8 (32.1) | 112.8 (37.3) | 105.1 (39.1) | <.001 |
| HDL-C, mean (SD), mg/dL | 54.3 (12.1) | 51.3 (12.6) | 52.4 (14.5) | 47.2 (9.8) | .001 |
| Triglycerides, mean (SD), mg/dL | 130.3 (69.7) | 126.6 (66.6) | 120.6 (50.2) | 169.6 (175.6) | .005 |
| Glomerular filtration, mean (SD), mL/min | 78.9 (21.6) | 72.8 (22.5) | 74.4 (27.3) | 68.9 (17.5) | .001 |
| LVH on ECG* | 53 (12.3) | 56 (18.2) | 23 (20.7) | 8 (20.5) | .047 |
| Statins | 96 (22.3) | 73 (23.8) | 34 (30.6) | 22 (56.4) | <.001 |
| Antiplatelet agents | 41 (9.5) | 69 (22.5) | 40 (36) | 27 (69.2) | <.001 |
BMI, body mass index; DAP, diastolic arterial pressure; ECG, electrocardiogram; HDL-C, high-density lipoprotein cholesterol; HT, hypertension; LDL-C, low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; SAP, systolic arterial pressure; SD, standard deviation.
Unless otherwise indicated, the data are expressed as No. (%).
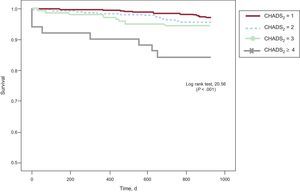
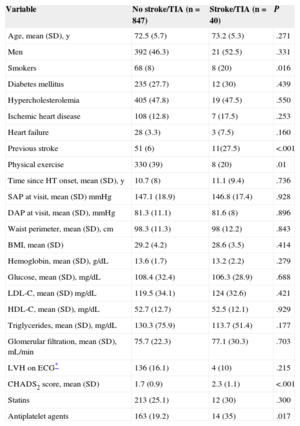
During follow-up, 40 (4.5%) patients required hospitalization for stroke/TIA, and the incidence was higher in patients with higher CHADS2 scores: 2.8% of CHADS2 1, 4.2% of CHADS2 2, 7.2% of CHADS2 3 and 17.9% of CHADS2 ≥ 4. The Kaplan-Meier curve in Figure 1 indicates poorer outcome in patients with higher CHADS2 scores. Patients with a cerebrovascular event showed a greater prevalence of smoking and previous stroke, and higher CHADS2 scores (2.3 [SD,1.1] vs 1.7 [SD,0.9]; P < .001) than those without this complication, in addition to practicing less physical exercise (Table 2). There were no differences in age or the prevalence of diabetes mellitus or hypercholesterolemia between the populations with and without an event over follow-up. Furthermore, patients who had a stroke/TIA were taking antiplatelet medication more often (35% vs 19.2%; P < .05), whereas there were no differences in the use of antihypertensive treatment or statins between the 2 populations.
Comparison Between Patients With and Without a Stroke/Transient Ischemic Attack Over Follow-up
| Variable | No stroke/TIA (n = 847) | Stroke/TIA (n = 40) | P |
|---|---|---|---|
| Age, mean (SD), y | 72.5 (5.7) | 73.2 (5.3) | .271 |
| Men | 392 (46.3) | 21 (52.5) | .331 |
| Smokers | 68 (8) | 8 (20) | .016 |
| Diabetes mellitus | 235 (27.7) | 12 (30) | .439 |
| Hypercholesterolemia | 405 (47.8) | 19 (47.5) | .550 |
| Ischemic heart disease | 108 (12.8) | 7 (17.5) | .253 |
| Heart failure | 28 (3.3) | 3 (7.5) | .160 |
| Previous stroke | 51 (6) | 11(27.5) | <.001 |
| Physical exercise | 330 (39) | 8 (20) | .01 |
| Time since HT onset, mean (SD), y | 10.7 (8) | 11.1 (9.4) | .736 |
| SAP at visit, mean (SD) mmHg | 147.1 (18.9) | 146.8 (17.4) | .928 |
| DAP at visit, mean (SD), mmHg | 81.3 (11.1) | 81.6 (8) | .896 |
| Waist perimeter, mean (SD), cm | 98.3 (11.3) | 98 (12.2) | .843 |
| BMI, mean (SD) | 29.2 (4.2) | 28.6 (3.5) | .414 |
| Hemoglobin, mean (SD), g/dL | 13.6 (1.7) | 13.2 (2.2) | .279 |
| Glucose, mean (SD), mg/dL | 108.4 (32.4) | 106.3 (28.9) | .688 |
| LDL-C, mean (SD) mg/dL | 119.5 (34.1) | 124 (32.6) | .421 |
| HDL-C, mean (SD), mg/dL | 52.7 (12.7) | 52.5 (12.1) | .929 |
| Triglycerides, mean (SD), mg/dL | 130.3 (75.9) | 113.7 (51.4) | .177 |
| Glomerular filtration, mean (SD), mL/min | 75.7 (22.3) | 77.1 (30.3) | .703 |
| LVH on ECG* | 136 (16.1) | 4 (10) | .215 |
| CHADS2 score, mean (SD) | 1.7 (0.9) | 2.3 (1.1) | <.001 |
| Statins | 213 (25.1) | 12 (30) | .300 |
| Antiplatelet agents | 163 (19.2) | 14 (35) | .017 |
BMI, body mass index; DAP, diastolic arterial pressure; ECG, electrocardiogram; HDL-C, high-density lipoprotein cholesterol; HT, hypertension; LDL-C, low-density lipoprotein cholesterol; LVH, left ventricular hypertrophy; SAP, systolic arterial pressure; SD, standard deviation; TIA, transient ischemic attack.
Unless otherwise indicated, the data are expressed as No. (%).
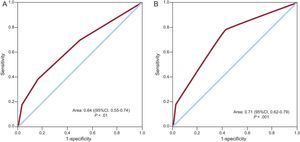
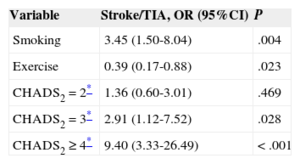
On multivariate analysis, the factors associated with the incidence of stroke/TIA were smoking and the CHADS2 score, with a higher risk in patients with values of 3 or greater (Table 3). In contrast, physical exercise was associated with a lower risk of stroke/TIA. The area under the ROC curve of the CHADS2 score for the risk of stroke/TIA was 0.64 (95% confidence interval [95%CI], 0.55-0.74; P < .01) (Figure 2A). In the light of these results, we also calculated the ROC curve of the combined score of significant variables in the multivariate analysis (CHADS2 + smoking + sedentary lifestyle) for the risk of stroke/TIA, which yielded an area under the curve of 0.71 (95%CI, 0.62-0.79; P < .001) (Figure 2B).
Multivariate Analysis. Factors Associated With the Appearance of a Stroke/Transient Ischemic Attack Over Follow-up
| Variable | Stroke/TIA, OR (95%CI) | P |
|---|---|---|
| Smoking | 3.45 (1.50-8.04) | .004 |
| Exercise | 0.39 (0.17-0.88) | .023 |
| CHADS2 = 2* | 1.36 (0.60-3.01) | .469 |
| CHADS2 = 3* | 2.91 (1.12-7.52) | .028 |
| CHADS2 ≥ 4* | 9.40 (3.33-26.49) | < .001 |
OR, odds ratio; TIA, transient ischemic attack.
The variables introduced in the model were sex, smoking, hypercholesterolemia, ischemic heart disease, physical exercise, systolic and diastolic arterial pressure, time elapsed since hypertension onset, waist perimeter, glomerular filtration rate, body mass index, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, diuretics, calcium channel blockers, statins, antiplatelet agents, and CHADS2 score.
This is one of the first studies evaluating the prognostic value of the CHADS2 score to estimate the risk of a cerebrovascular event in a cohort of hypertensive patients without known atrial fibrillation from a Mediterranean area. The results show that CHADS2 is a good predictor of stroke/TIA, such that patients with a score of 3 or greater have a higher risk of experiencing a cardiovascular event at mid-term.
Atherosclerotic cardiovascular disease, particularly cerebrovascular disease, is one of the main causes of premature death and disability in industrialized countries.9 The development and progression of atherosclerotic disease is often insidious, and it can manifest in advanced stages without previous warning symptoms. Hence, it is important to establish the risk of stroke and provide adequate medical treatment to reduce the high economic burden placed by these diseases on the health system. In the last few years, use of the CHADS2 score has extended beyond the original scenario of atrial fibrillation,14,15 and it has shown certain advantages over other methods (SCORE or Framingham criteria), such as inclusion of older patients and greater ease of use in daily practice. Henriksson et al applied this score to a large series of stroke survivors included in the Swedish Stroke Registry and reported that the risk of death due to a cerebral event at 5 years showed a progressive, linear increase in parallel with the CHADS2 score, in both patients with atrial fibrillation and those in sinus rhythm.16 These data were recently confirmed in other studies, showing a higher incidence of mortality, recurrent stroke, and cardiovascular events in stroke patients with a CHADS2 score of 2 or greater, regardless of whether they had atrial fibrillation.17,18
The role of CHADS2 has also been investigated in ischemic heart disease. In a study in 916 patients who were not receiving anticoagulants, with stable coronary disease and no atrial fibrillation, patients with an intermediate (2-3) or high (4-6) CHADS2 score had a higher risk of stroke/TIA than those with a low (0-1) score, after adjustment had been made for other risk factors.19 Furthermore, the incidence of stroke in ischemic patients with a score above 5 was comparable to the reported rate in patients with atrial fibrillation and CHADS2 1 or 2, a population known to benefit from stroke prevention therapies, such as anticoagulation.20 The prognostic value of the score has also been demonstrated in patients with an acute coronary syndrome and no atrial fibrillation. High CHADS2 scores at hospital admission were associated with a higher risk of hospitalization due to stroke and greater mortality during follow-up.21 More recently, the CHADS2 score showed an ability to predict stroke in patients who had undergone pacemaker implantation for sinus node disease.22
In the present study, we have extended the ambit in which this score can be used to the field of hypertension, the most important factor determining stroke risk. We found an association between CHADS2 results and the mid-term risk of experiencing a stroke in a sample of hypertensive patients aged 65 years or older. Risk progressively increased in parallel with the CHADS2 value, such that patients with a score of 4 or greater had a 9-fold higher risk of having a cerebrovascular event than those with a score of 1. We believe that these novel findings in this high-risk population may provide valuable support for the use of this straightforward, easily applied predictive scheme in our setting.
Several potential mechanisms may explain the ability of CHADS2 to predict stroke risk in hypertensive patients without atrial fibrillation. First, patients with a higher CHADS2 score can have a greater risk of atrial arrhythmia. A study performed in hospitalized ischemic stroke patients undergoing monitoring showed a higher incidence of episodes of occult atrial fibrillation in those with higher CHADS2 scores.23 Second, the various risk factors comprising CHADS2 may, in themselves, increase the risk of stroke, independently of the cardiac rhythm. In patients with heart failure24 and diabetes mellitus,25 plasma markers of hypercoagulation and endothelial dysfunction are elevated, and these mechanisms are implicated in thrombus formation and stroke in patients with atrial fibrillation.26 Lastly, the various components of the CHADS2 score may directly contribute to left atrial remodeling, a process characterized by atrial dilatation and mechanical dysfunction.27 This may lead to blood stasis and an increased thromboembolic risk regardless of the cardiac rhythm.28 In this line, a recent study in 970 patients with coronary disease reported an association between the CHADS2 score and the functional score, an echocardiographic parameter of left atrial dysfunction, even in patients without atrial fibrillation, opening debate on the role of left atrial dysfunction in cardioembolic stroke.29
LimitationsOne of the limitations of this study is selection bias. The patients enrolled spontaneously attended a medical visit; therefore, the study conclusions cannot be extrapolated to other settings. Furthermore, because the events included were obtained from an analysis of hospital admissions, an indeterminate number of TIA patients who did not consult were not detected in the analysis. Stroke and TIA were analyzed in a global manner and no distinction was made among the various causes of these conditions (embolic, atherothrombotic, lacunar, etc.). Finally, the study lacks a second, independent cohort to validate the predictive results obtained in the sample.
CONCLUSIONSOur findings indicate that the CHADS2 score, a fast, simple, and easy to use tool, may have a role in estimating the risk of a cerebrovascular event in hypertensive patients without known atrial fibrillation. In addition, our data raise the question of whether patients with higher CHADS2 scores might benefit from preventive therapies such as anticoagulation because of their higher risk of silent atrial fibrillation30 or thromboembolic mechanisms independent of the heart rhythm. Studies investigating this possibility could be warranted.
CONFLICT OF INTERESTSNone declared.
We thank Lácer Laboratories for their disinterested help with this project and the participating investigators for their invaluable contribution. Without their work and effort, this study would not have been possible.
Juan Alberola, Vicente Javier; Maestre Amat, Luis; Mateo Limiñana, Jose Manuel; Monleon Gomez, Jose; Montagud Moncho, Miguel; Guinot Martinez, Enrique; Gamon Pastor, Jose Blas; Salanova Penalba, Alejandro; Sanchis Domenech, Carlos; Pallares Carratala, Vicente; Palacios del Cerro, Antonio; Perez Martinez, Rafael; Baudet Dejean, Chantal; Perez Alonso, Manuel; Facila Rubio, Lorenzo; Sipan Sarrion, Yolanda; Saro Perez, Eugenia; Villaro Gumpert, Juan; Cabrera Ferriols, M. Angeles; Fraile Fraile, Belen; Carbonell Franco, Francisco; Cornejo Mari, Francisco Javier; Barbera Comes, Javier; Quiles Añon, Fernando; Llisterri Caro, Jose Luis; Almenar Cubells, Enrique; Casado Gonzalez, Joaquin; Godoy Rocati, Diego; Martinez Guerola, Carmen; Bonet Garcia, Jorge Alejo; Blazquez Encinar, Julio Cesar; Botella Estrada, Carlos; Saen Alcoy, Montepio; Almarcha Perez, Natividad; Salanova Chilet, Lorena; Torres Ferrando, Miquel; Debon Belda, Manuel; Fluixa Carrascosa, Carlos; Aznar Baset, Lucia; Vivancos Aparicio, Diego; Pineda Cuenca, Manuel; Obarrio Moreno, Alicia; Nuñez Jorge, Carlos; Matoses Nacher, Daniel; Baño Aracil, Manuel; Balanza Garzon, Alicia; Garcia Palomar, Carlos; Peña Forcada, Enrique; Raga Casasus, Jose; Martinez Lahuerta, Juan; Mendizabal Nuñez, Andrea; Santos Alonso, Eufrosina; Corbi Pascual, Miguel; Lillo Sanchez, Antonio; Martorell Adsuara, Vicente; Sanchez Ruiz, Tomas; Ortiz Diaz, Francisco; Llinares Orts, Jose Francisco; Lahoz Ferrer, Julio; Morillas Blasco, Pedro; Pertusa Martinez, Salvador; Manclus Montoya, Carlos; Adria Mico, Jose Manuel; Llaudes Soler, Ricardo; Castillo Castillo, Jesus; Llopis Martinez, Francisco; Ruiz de la Prada Abarzuza, Ignacio; Nebot Rico, Lidia.