Area at risk (AAR) quantification is important to evaluate the efficacy of cardioprotective therapies. However, postinfarction AAR assessment could be influenced by the infarcted coronary territory. Our aim was to determine the accuracy of T2-weighted short tau triple-inversion recovery (T2W-STIR) cardiac magnetic resonance (CMR) imaging for accurate AAR quantification in anterior, lateral, and inferior myocardial infarctions.
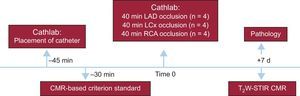
MethodsAcute reperfused myocardial infarction was experimentally induced in 12 pigs, with 40-minute occlusion of the left anterior descending (n = 4), left circumflex (n = 4), and right coronary arteries (n = 4). Perfusion CMR was performed during selective intracoronary gadolinium injection at the coronary occlusion site (in vivo criterion standard) and, additionally, a 7-day CMR, including T2W-STIR sequences, was performed. Finally, all animals were sacrificed and underwent postmortem Evans blue staining (classic criterion standard).
ResultsThe concordance between the CMR-based criterion standard and T2W-STIR to quantify AAR was high for anterior and inferior infarctions (r = 0.73; P = .001; mean error = 0.50%; limits = −12.68%-13.68% and r = 0.87; P = .001; mean error = −1.5%; limits = −8.0%-5.8%, respectively). Conversely, the correlation for the circumflex territories was poor (r = 0.21, P = .37), showing a higher mean error and wider limits of agreement. A strong correlation between pathology and the CMR-based criterion standard was observed (r = 0.84, P < .001; mean error = 0.91%; limits = −7.55%-9.37%).
ConclusionsT2W-STIR CMR sequences are accurate to determine the AAR for anterior and inferior infarctions; however, their accuracy for lateral infarctions is poor. These findings may have important implications for the design and interpretation of clinical trials evaluating the effectiveness of cardioprotective therapies.
Keywords
In acute myocardial infarction (MI), the ischemic area at risk (AAR) is a major determinant of infarct size and clinical outcome.1,2 In fact, mortality is reduced by increasing the size of the salvaged myocardium, defined as the proportion of AAR that does not become necrotic, using early coronary reperfusion strategies.3 Therefore, accurate quantification of AAR and salvaged myocardium is of tremendous value in clinical trials testing the efficacy of therapies aimed at reducing infarct size.4
Cardiac magnetic resonance (CMR) has emerged as the best noninvasive technique for characterizing post-MI myocardium status. In particular, the T2-weighted short tau triple-inversion recovery (T2W-STIR) sequence is the most widespread CMR sequence to determine AAR since it nicely highlights edema secondary to myocardial ischemia/reperfusion.5–7 Previous experimental8,9 and clinical10,11 examinations have evaluated the value of T2W-STIR for AAR quantification by comparing it with classic criterion standards: histopathology or other imaging modalities able to grossly identify AAR, such as single-photon emission computed tomography.12 However, histopathology is a terminal postmortem evaluation and the low spatial resolution of single-photon emission computed tomography precludes accurate evaluation. Cardiac magnetic resonance perfusion imaging during selective intracoronary injection of contrast media could be an appropriate criterion standard because it can accurately delineate in vivo the myocardial area irrigated by a coronary artery distal to the perfusion site without radiation exposure13; however, it has not been validated against a proper histological criterion standard.
It is not known whether different coronary infarcted territories influence the accuracy of T2W-STIR imaging for AAR assessment. Recently, our group demonstrated that edema is not stable during the first week after reperfusion in an ischemia/reperfusion swine model.14 There is a bimodal pattern with a first wave of reperfusion-related edema occurring upon reperfusion that exhausts at 24hours and a second wave of edema secondary to tissue repair/healing.15 This second wave of edema progressively appears several days later and peaks at day 7. Nevertheless, the influence of the coronary territory infarcted for AAR quantification remains unclear. Our aim was therefore to evaluate the utility of T2W-STIR imaging performed at day 7 when acute MI affects different myocardial territories.
METHODSStudy DesignA reperfused acute MI was experimentally induced in 12 three-month-old castrated large male white pigs by using an angioplasty-based ischemia/reperfusion model. Acute MI was created as previously described.16–18 Continuous electrocardiography, blood pressure monitoring, and pulse oximetry were available for all animals. Anesthesia was induced by intramuscular injection of ketamine (20mg/kg), xylazine (2mg/kg), and midazolam (0.5mg/kg) and was maintained by intravenous administration of midazolam (0.2mg/kg/h). Buprenorphine (0.3mg/kg) was used for analgesia. All animals were intubated and mechanically ventilated with oxygen (FiO2, 28%). Continuous infusion of amiodarone (300mg, 150mg/h) was maintained during the procedure as prophylaxis for malignant ventricular arrhythmias. The animals were anticoagulated by 300 IU/kg heparin administration (an activated clotting time was above 250seconds in all cases).
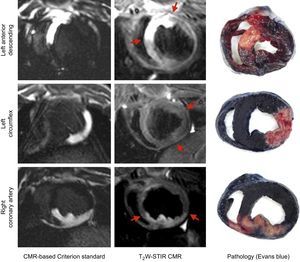
The study design is summarized in Figure 1. The percutaneous procedure included a femoral arterial 6-F line and an over-the-wire coronary angioplasty balloon (Voyager, Abbott) that was placed in the midleft anterior descending artery (LAD) (n = 4), in the proximal left circumflex (LCx) (n = 4) or in the proximal right coronary artery (RCA) (n = 4). As in previous ischemia/reperfusion models in our group,17,18 the LAD was occluded after the first diagonal branch to reduce mortality secondary to arrhythmias or cardiogenic shock compared with proximal LAD occlusions while still resulting in large infarctions. After a coronary angiogram confirmed correct catheter position, the pigs were carefully taken to the CMR suite located wall-to-wall with the experimental catheterization laboratory. An in vivo perfusion CMR-based criterion standard was performed during selective intracoronary gadolinium injection at the subsequent coronary occlusion site to delineate the AAR () and the balloon was then inflated to induce an acute MI for 40minutes. After 7 days post-MI, a follow-up CMR was performed and subsequently all animals were sacrificed to validate the CMR-based criterion standard against classic Evans blue staining. All animals received experimental care in accordance with the Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Research Committee at the Spanish National Center for Cardiovascular Research (CNIC).
Cardiac Magnetic Resonance Imaging ProtocolAll studies were performed using a 3 Tesla (3T) Philips Achieva-TX whole body scanner (Philips Healthcare, Medical Systems, Best, The Netherlands) equipped with a 32-element cardiac phased-array surface coil using electrocardiographic gating. Anesthesia was induced and maintained as described above in each study. In the 7-day post-MI CMR, the following procedures were performed: SSFP sequences for function (11-13 contiguous left ventricle (LV) short-axis slices; field of view 280 x 280mm; slice thickness 8mm without gap; echo time/repetition time 1.4/2.8ms, flip angle 45°; cardiac phases 25; voxel size 1.8 x 1.8mm; SENSE factor 1.5; NEX 3) and black blood T2W-short tau triple-inversion recovery (T2W-STIR) sequences for edema (11-13 contiguous LV short-axis slices; field of view 280 x 280; slice thickness 8mm without gap; TE 80ms; voxel size 1.4 x 1.4mm; echo-train length 16; NEX 2). A coil sensitivity correction algorithm for all T2W images was implemented in the scan acquisition. The T2W-STIR sequence was tuned for the 3T scanner by adjusting the inversion time to 200ms to correct from longest T1 at 3T and the acquisition was triggered for every 2 beats to avoid signal saturation due to very short repetition time. Homogenous excitation was ensured by multichannel excitation technology. Thus, the results reported here are applicable to 1.5 T scanners.
In addition, a CMR-based AAR criterion standard was acquired before MI induction using perfusion imaging (field of view 280 x 280mm; slice thickness 8mm without gap; flip angle 15°; TR 2.8ms; TE 1.4ms; saturation time 45ms; voxel size 2.4 x 2.4) and inversion recovery gradient-echo sequences as those typically used for scar assessment (11-13 contiguous short-axis slices; field of view 280 x 280mm; slice thickness 8mm without gap; flip angle 15°; TR 5.6ms; TR 2.8ms; voxel size 1.6 x 1.6mm; inversion time 500ms, shot interval, 2 beats) during selective coronary injection of gadolinium. Inversion recovery images were obtained in addition to intracoronary gadolinium perfusion images to improve the spatial resolution for border delineation. Preliminary experiments were performed to determine the best intracoronary gadolinium concentration and infusion volume/rate. Accordingly, all animals received 0.2 mmoL/kg gadolinium-based contrast media through the coronary catheter at 2mL/s for intracoronary gadolinium perfusion and at 0.15mL/s for inversion recovery imaging. All short-axis slices matched those of the cine imaging within the same study and, similarly, follow-up images were done with the same slice thickness and position as baseline according to anatomical landmarks.
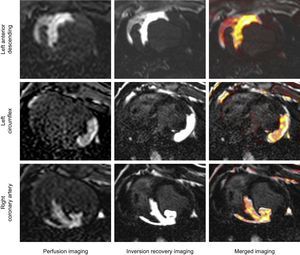
Cardiac Magnetic Resonance Postprocessing AnalysisCardiac magnetic resonance images were analyzed using dedicated software (QMASS 7.5, Medis, Leiden, The Netherlands). Basal LV slices proximal to the site of coronary artery occlusion without AAR or apical LV slices with slow flow and/or partial volume artifacts were excluded. Border delineation on the CMR-based criterion standard for anatomical AAR delineation was mainly performed on inversion recovery images due to better contour visualization. However, special care was taken to confirm concordance between the extent of hyperenhanced areas on intracoronary gadolinium perfusion and inversion recovery images (Figure 2). The epicardial and endocardial contours in each LV short axis were manually traced including papillary trabeculations within the cavity. Areas at risk on T2W-STIR images were identified as hyperenhanced regions using the full-width half-maximum method8 and the extent of AAR was calculated with respect to the total LV slice area (%). Extreme care was taken to avoid including any artificially high signal intensity due to inadequately suppressed slow flow within the cavity space. If present, a central core of hypointense signal within the area of increased signal was included in the T2W-STIR analysis. Additionally, regions of interest sized 0.5mm2 were manually drawn on T2W-STIR images to calculate the average signal intensity for all pixel values within the hyperenhanced area (AAR) and the normal signal area (remote myocardium). Hypointense areas within the AAR in cases of microvascular obstruction or hemorrhage were carefully avoided. Then, the signal intensity change between the AAR and remote myocardium for each study was calculated as (signal intensityAAR – signal intensityremote)/signal intensityremote.9,19
HistopathologyAll animals were transferred to the catheterization laboratory for AAR assessment by Evans blue staining after the 7-day CMR study. The LAD (n = 4), LCx (n = 4) or RCA (n = 4) was reoccluded by angioplasty balloon inflation at the same level as the initial occlusion, following the same angiographic landmarks. Then, 100mL of Evans blue dye (5%) were infused into the LV cavity through a pigtail catheter while the balloon was inflated. With this technique, nonischemic areas appear blue, whereas the AAR remains unstained.20 The animals were then euthanized with a lethal injection of pentobarbital sodium after heparin administration and their hearts were excised. After 3hours of freezing, the hearts were sliced into 8-mm section slices using a commercial rotator blade, and midventricular consecutive slices were selected as they matched to the 8-mm short axis CMR images of interest. Finally, high-resolution photographs were taken using a digital camera (Pentax K-m) and ImageJ software v.1.45s (National Institutes of Health, Bethesda, Maryland, United States) was used for AAR quantification. The AAR was expressed as a percentage of the total slice LV area (%) after manual delineation of cardiac contours.
Statistical AnalysisContinuous variables are expressed as mean ± standard deviation. The correlations between the measures were assessed by Spearman rank order coefficients and Bland-Altman plots were used to study the agreement. To take into account repeated measures and clustering (several slices by animal), analyses were performed using the mixed-model procedure. A P value < .05 was considered statistically significant. All statistical tests were performed with IBM SPSS Statistics software, v.20.0 (SPSS, Inc, Chicago, Illinois).
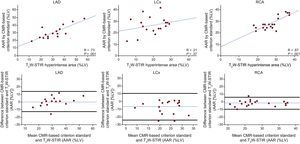
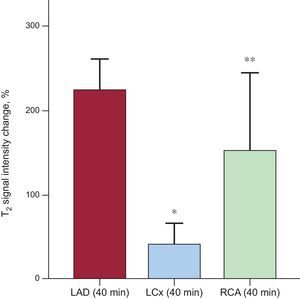
RESULTSCapacity of T2-weighted Short tau Triple-inversion Recovery to Accurately Quantify Area at Risk in Different Coronary TerritoriesThe agreement between the CMR-based criterion standard and T2W-STIR in the different coronary artery territories is summarized in Figure 3. The correlation between the CMR-based criterion standard AAR delineation and T2W-STIR-based AAR quantification for LAD and RCA infarctions was high, with an r coefficient of 0.73 (P = .001), mean error 0.50% and limits of agreement of −12.68% and 13.68% for LAD infarctions and an r coefficient of 0.87 (P = .001), mean error of −1.5% and agreement limits of −8.0% and 5.8% for RCA infarctions. Conversely, the correlation for LCx territories was poor (r = 0.21; P = .37), showing a higher mean error and wider limits of agreement for this territory. Similar results were obtained on comparison of the classic histopathology criterion standard and T2W-STIR images (). In terms of intensity values, statistically significant differences were found between LAD and RCA infarctions (224.5 ± 36.5% vs 153.1 ± 90.3%; P = .003), and the differences were even higher between LAD and LCx infarctions (224.5 ± 36.5% vs 41.7 ± 23.1%; P < .001) (Figure 4).
Agreement between the CMR-based criterion standard and T2W-STIR images according to each coronary artery territory in animals undergoing 40minutes of ischemia/reperfusion. Spearman correlation is shown in upper panels and Bland-Altman plots in lower panels where the blue line represents the mean error and black lines the limits of agreement. AAR, area at risk; CMR, cardiac magnetic resonance; LAD, left anterior descending artery; LCx, left circumflex artery; LV, left ventricle, RCA, right coronary artery; T2W-STIR, T2-weighted short tau triple-inversion recovery.
T2W-STIR signal intensity values in animals with ischemia/reperfusion affecting different ventricular territories. Statistically significant differences were found between the signal intensity values for infarctions affecting the LAD (224.5 ± 36.5%) and RCA (153.1 ± 90.3%); P = .003. Differences between LAD and LCx infarctions were even higher (224.5 ± 36.5% vs 41.7 ± 23.1%; P < .001). Bars represent mean ± standard deviation. LAD, left anterior descending artery; LCx, left circumflex, RCA, right coronary artery; T2W-STIR, T2-weighted short tau triple-inversion recovery. *P < .001. **P = .003 for comparisons with the LAD Group.
There was highly significant good correlation (r = 0.84; P < .001) and agreement (mean error = 0.91%; limits −7.55%-9.37%) between the extent of AAR as measured by the CMR-based criterion standard (intracoronary gadolinium perfusion) and by the histological Evans blue evaluation (Figure 5). Similarly, the extent, shape, and contours of the hyperintense area depicted by both standards and by T2W-STIR images were strongly concordant for each of the coronary artery territories (Figure 6).
Concordance between CMR-based criterion standard and classic histological standard (negative Evans blue staining) for AAR delineation. Left panel shows the scatter plot and right panel illustrates Bland-Altman plots where blue line represents the mean error and black lines the limits of agreement. AAR, area at risk; CMR, cardiac magnetic resonance; LV, left ventricle.
In recent years, there has been growing interest in the possibility of measuring post-MI AAR to assess salvaged myocardium. Several studies have shown that T2W-STIR CMR sequences can depict the edematous post-MI region, corresponding to the ischemic zone; however, there have been no reports of the potential influence of the infarcted coronary territory. We performed a comprehensive CMR study in a pig model of ischemia/reperfusion and found that T2W-STIR CMR accurately quantified AAR when the anterior or inferior myocardial territories are infarcted; however, it does not accurately quantify AAR in the lateral LV wall (resulting from LCx infarction). These findings have implications for the design and interpretation of clinical trials using post-MI AAR and salvage index by CMR as endpoints.
Although previous experimental and clinical studies have supported the use of T2W-CMR to delineate AAR,8 the reference method described in those studies has some inherent limitations. We decided to validate an in vivo criterion standard (intracoronary gadolinium perfusion) to delineate the actual myocardial area that will later undergo ischemia. This approach has been previously used by other authors to assess the effect of novel therapies in a specific LV wall13 and to quantify AAR in anterior nonreperfusion MI21; however, a proper validation against the histological classic criterion standard was lacking. We have validated this novel methodology for the 3 main coronary arteries and the results were significantly correlated against histology. The possibility of having an accurate in vivo criterion standard for AAR delineation is of great value for longitudinal serial studies, allowing a significant reduction in the number of experiments by eliminating intermediate histological validations.
T2-weighted short tau triple-inversion recovery imaging is not homogeneous across all the LV walls. Indeed, T2W-STIR imaging of lateral ventricular regions is particularly challenging because of signal intensity loss secondary to through-plane cardiac motion in late-diastole or possible susceptibility effects due to air in the lungs causing the CMR signal to decay faster.22,23 Although this phenomenon is widely acknowledged, there has been no previous evaluation of whether edema can be quantified with equal accuracy in different ventricular regions. Thus, to our knowledge, this is the first study to compare the accuracy of T2W-CMR AAR evaluation in different MI territories against a standard method. We demonstrate that T2W-STIR accurately quantifies AAR in the anterior and inferoposterior walls, but quantification of AAR in the lateral wall (resulting from LCx infarction) showed poor correlation with the criterion standard. This poor correlation was accompanied by consistently and significantly lower signal intensity values compared with other myocardial territories, despite the use of coil sensitivity reference scans (CLEAR) and a multitransmit CMR system to ensure a homogeneous signal throughout the heart. This is a key and novel parameter to take into account since low signal intensity of the affected myocardium may limit the proper identification of AAR boundaries and thus AAR delineation.
The time course of edema formation has recently been evaluated by our group.14,15 Given that the second wave of edema seems to peak around day 7 post-MI, we decided to perform the CMR studies at this moment. Edema extent at earlier time points might be smaller and thus potentially result in poorer correlation with the criterion standard. Nevertheless, this is speculative at this time since it has not been specifically tested to date.
LimitationsWe used a black blood T2W-STIR sequence for edema depiction. Alternative sequences (T2W-bright blood sequences, T2P-SSFP, or T2 and T1 mapping) have recently been proposed for imaging myocardial edema and could potentially overcome the limitations of T2W-STIR for AAR quantification in the lateral wall. However, T2W-STIR imaging is currently the most commonly used method to assess the presence and extent of myocardial edema both in the clinical scenario24 and, more importantly, in clinical trials with the salvage index as endpoint.25–29 Given that this study was designed to explore the accuracy of the classic T2W-STIR imaging for AAR quantification according to the MI territory, T2-mapping sequences were not included in the imaging protocol. Measurements of collateral flow were not available in this study, which could have influenced the AAR size. However, the swine model of acute reperfused MI is known to have very little or no collateral flow.30 This is an experimental model with a small sample (n = 12) and thus human validation studies are needed to confirm our findings.
CONCLUSIONSIn a swine model of reperfused MI with an in vivo CMR-based reference standard, we have shown that infarctions affecting the lateral wall are limited for AAR delineation by T2W-STIR CMR imaging compared with a proper evaluation of anterior or inferior MI. These data might have a significant impact on the design and interpretation of clinical trials that have the salvage index as an endpoint. Validation with a larger sample size or in clinical studies would provide incremental value to our results.
FUNDINGThis work was funded by grants from the Spanish Ministry of Economy and Competitiveness (MINECO) through the Instituto de Salud Carlos III of Health Fondo de Investigación Sanitaria and ERDF/FEDER programs (PI13/01979 and RETIC# RD12/0042/0054) to B. Ibáñez. J.M. García-Ruiz and A. García-Álvarez were CNIC-cardiojoven fellows. R. Fernández-Jiménez was the recipient of nonoverlapping grants from the Ministry of Economy and Competitiveness through the Instituto de Salud Carlos III (Rio Hortega fellowship); and the Fundación Jesús Serra, the Fundación Interhospitalaria de Investigación Cardiovascular (FIC) and the CNIC (FICNIC fellowship). The CNIC is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the Pro-CNIC Foundation, and is a Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505). B. Ibáñez is Princess of Girona awardee in science.
CONFLICTS OF INTERESTJ. Sánchez-González is employee of Philips Healthcare.
- -
Myocardial salvage, which is based on AAR quantification, is an important determinant of clinical outcomes in myocardial infarction and has been used to evaluate the effectiveness of cardioprotective therapies. Although different techniques can be used to assess AAR, such as single-photon emission computed tomography or histology, magnetic resonance imaging is considered the technique of choice. However, there is still controversy about the ability of magnetic resonance imaging, especially of T2W-STIR sequences, to accurately quantify AAR according to the affected myocardial territory. Currently, T2W-STIR is used in clinical trials for quantifying myocardial salvage including infarctions at any location.
- -
For the first time, this study shows the limited value of T2W-STIR to quantify AAR in lateral infarctions. This has important implications for the design of clinical trials assessing cardioprotective therapies. This study suggests that T2W-STIR accurately quantifies AAR when the affected territory is the anterior or inferior ventricular area.
- -
In this study, a new in vivo criterion standard by magnetic resonance imaging is proposed and validated to evaluate AAR. This new standard has added value to the classic histological method since it is based on the same imaging modality