Keywords
GENERAL CONSIDERATIONS
Over 75 years ago, Saunders1 described the clinical triad of arterial hypotension, elevated jugular venous pressure, and clear lung fields in a patient with extensive necrosis of the right ventricle and minimal involvement of the left ventricle. Acute right ventricular infarction (ARVI) is now diagnosed through electrocardiography (ECG) recordings from the right-chest leads. Also, the clinical prevalence, requirement for specific treatment, and prognostic implications of the condition are currently underestimated.
Historically, ARVI was not considered an important manifestation of acute ischemic coronary syndromes; this was due in part to the finding that isolated right ventricular infarction in experimental animals did not lead to significant changes in systemic venous pressure or in pulmonary arterial pressure.2
Experimental studies in dogs led to identification of the pathophysiology of reduced cardiac output following occlusion of the right coronary artery; a disproportionate increase was observed in the filling pressure of the right heart chambers compared with that of the left chambers, along with an increase in the size of the right ventricle and a reduction in the dimensions of the left ventricle.3,4
The association of ARVI with inferior wall infarction of the left ventricle has been observed in 10% to 50% of patients, according to noninvasive, hemodynamic, or postmortem diagnostic criteria.5,6
The right ventricle is a crescent moon-shaped chamber with a myocardial mass approximately a sixth that of the left ventricle, and since pulmonary vascular resistance corresponds to a tenth of the systemic vascular resistance, it performs a quarter of the work per beat of the left ventricle.7 In addition, there is an interdependence between the ventricles, due to their sharing an interventricular septum and surrounding pericardium, and they have a similar cardiac output.5,7
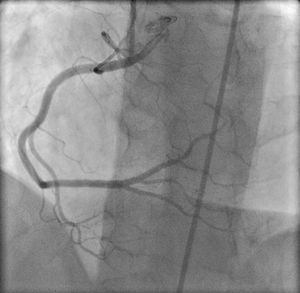
The right coronary artery (RCA) mainly provides blood flow to the right ventricular myocardium; the conal branch of the RCA irrigates the outflow tract and the acute marginal branches irrigate the posterior wall of the right ventricle. According to the coronary artery dominance profile, the posterior descending artery may irrigate to varying degrees the posterior wall of the left ventricle. The left coronary artery usually provides little blood flow to the anterior wall of the right ventricle via small branches of the anterior descending artery (Figure 1).
Figure 1. Normal right coronary artery. Initially, the artery generates branches to the right atrium, the conal branch, which irrigates the right ventricular outflow tract, and the sinus node artery. The acute marginal branches to the posterior wall. Atrioventricular node artery. The posterior descending artery generates septal perforating arteries that supply the posterobasal segment of the interventricular septum.
Blood flow to the right atrial myocardium is provided by small branches arising from the proximal portion of the RCA; supplementary flow is occasionally provided by branches of the left circumflex artery.
ATRIAL INFARCTION
The first report of right atrial myocardial infarction detected by autopsy was provided by Clerc and Levy8 in 1925. In 1939, Langendorf9 reported an infarction of the right atrium observed in a postmortem study and retrospectively identified ECG changes compatible with atrial ischemia. In 1942, Cushing10 published clinical data and pathology findings from 182 patients who died as a result of ventricular myocardial infarction. Atrial infarction was demonstrated in 17% of the patients: 27 in the right atrium and 4 in the left. Mural thrombosis of the atrium was observed in 26 of the 31 cases of atrial infarction.
In 1948, Söderstrom11 analyzed 192 autopsies of patients with atrial thrombosis. Evidence of atrial myocardial infarction was found in 47 patients (24%) and in almost all of those cases (46 patients) it was localized to the right atrium. Two types of atrial infarction were described: type 1, or ventral, which is usually isolated and located in the atrial appendage or adjacent areas, and type 2, or dorsal, which usually involves an extensive area of the atrium and is associated with biventricular posteroinferior infarctions.
Between 81% and 98% of atrial infarctions are located in the right atrium. The associated complications include arrhythmias and pulmonary embolism, while hemodynamic deterioration may also appear due to loss of atrial contribution, and in exceptional cases wall rupture can occur.
PATHOPHYSIOLOGY
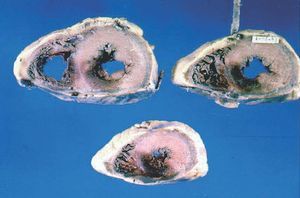
Inferior infarction of the right ventricle occurs through obstruction of the RCA proximal to the acute marginal branches. In patients with a dominant left coronary artery, occlusion of the circumflex artery can sometimes lead to right ventricular infarction. Another possibility, although very rare, is the generation of an infarction of the anterior wall of the right ventricle with proximal obstruction of the anterior descending artery. Based on the extent of myocardial necrosis in the right ventricle (Figure 2), Isner and Roberts12 described a classification that included 4 categories: grade I, when the necrosis covers less than 50% of the posterior wall of the right ventricle; grade II, when the infarction affects more than 50% of the posterior wall of the right ventricle; grade III, when the necrosis affects the posterior wall of the right ventricle and extends into less than 50% of the myocardium of the anterolateral wall; and grade IV, when the infarction includes the posterior wall and more than 50% of the anterolateral wall. In all 4 groups of that classification, the infarcted myocardium includes to greater or lesser degree the posterior portion of the interventricular septum.
Figure 2. Pathology specimen showing infarction in the posterior wall of the left ventricle (basal, medial, and apical) extending to posterior segments of the right ventricle (medial and apical)
According to the described classification, the larger the mass of right ventricular myocardium affected by necrosis, the greater the degree of hemodynamic abnormality and signs of infarction observed using noninvasive diagnostic methods. This classification does not take into account the spread of ischemia or necrosis to the myocardium of the right atrial wall, a situation that is not uncommon in patients with right ventricular infarction, with substantial hemodynamic deterioration and proximal occlusion of the right coronary artery.
A group of patients exists in whom, despite complete occlusion of the right coronary artery, left-sided infarction is not accompanied by significant necrosis of the right ventricle or right ventricular dysfunction. This has been associated with various factors, such as a) lower oxygen demand in the right ventricular myocardium; b) much higher systolic-diastolic coronary blood flow ratio in the arteries that perfuse the right ventricle13; c) greater capacity of the right ventricle to extract oxygen under conditions of hemodynamic stress14; d) probable direct perfusion from the heart chamber to the right ventricular myocardium through the thebesian veins15; and e) the presence of extensive coronary collateral circulation.16 In this regard, it has been demonstrated in postmortem studies that 75% of the samples with right ventricular necrosis have more than 75% obstruction of the anterior descending artery, a finding which indicates that loss of sufficient left-to-right coronary collateral circulation could be involved in the pathogenesis of right ventricular infarction.12,17 A protective role for collateral circulation has been proposed to explain the low incidence of right ventricular infarction in patients with inferior left ventricular infarctions and history of angina.18
Patients with ARVI have right ventricular systolic and diastolic dysfunction. Systolic dysfunction is manifested by reduced cardiac output and arterial hypotension, while diastolic dysfunction is displayed as a disproportionate increase in right ventricular filling pressures compared with those for the left ventricle.19-22 Reduced left ventricular compliance causes a) increased right atrial pressure; b) increased right ventricular filling pressure during inspiration (Kussmaul's sign); and c) a "noncompliant" profile in the pressure curve for the right atrium, characterized by A and V waves with equal amplitudes (reduced A wave), caused by descent of the X and Y waves. These abnormalities in the pressure curves of the right heart chambers do not only indicate a reduction in diastolic compliance of the right atrial and ventricular myocardium; they also reflect a constrictive effect of the pericardium secondary to acute distension of the right ventricle.
The hemodynamic criteria for ARVI were established by López-Sendón et al,23 who correlated hemodynamic data from 60 patients with the presence of right ventricular necrosis. A right atrial pressure of more than 10 mm Hg and a ratio of right atrial to pulmonary capillary pressure greater than 0.86 indicate ARVI with a sensitivity of 82% and a specificity of 97%. The low sensitivity of these criteria has been linked to the presence of left ventricular dysfunction and its effect on pulmonary capillary pressure. The hemodynamic criteria for ARVI may not be met in patients with left inferior infarction and may only be apparent after rapid administration of a volume load (saline).24
An increase in pressure in the right chambers until it equals that of the left chambers is not specific to ARVI. The differential diagnosis includes diseases that predominantly affect diastolic function, such as cardiac tamponade, constrictive pericarditis, restrictive cardiomyopathy, or diseases that compromise systolic function, such as thromboembolic pulmonary hypertension.25
Experimental and human studies have shown that an increase in right atrial contraction improves right ventricular filling flow, ventricular systolic function, and cardiac output.26,27 In contrast, the lack of right atrial mechanical contribution reduces ventricular filling and accentuates the hemodynamic effects of right ventricular dysfunction. It is important to remember that in the absence of right atrial myocardial ischemia or asynchronous atrioventricular activation, increased right atrial contractility is a compensatory mechanism for ischemic right ventricular dysfunction.
CLINICAL PRESENTATION AND PHYSICAL EXAMINATION
Prompt diagnosis is important in patients with acute myocardial infarction (AMI) of the left inferior wall that extends to the right ventricle, especially in the presence of arterial hypotension or cardiogenic shock. Rapid identification of right ventricular infarction allows the use of diuretics or vasodilators to be avoided. The main clinical signs accompanying ARVI are a) jugular venous distension; b) absence of decreased jugular venous pressure on inspiration (Kussmaul's sign); c) arterial hypotension; d) bradycardia or atrioventricular block; e) tricuspid valve insufficiency; f) right ventricular gallop (S3 and S4); g) paradoxical pulse (reduction of more than 10 mm Hg in blood pressure on inspiration). The presence of jugular venous distension and Kussmaul's sign as an expression of ARVI has a high sensitivity (88%) and specificity (100%).28 In patients with inferior infarction with or without extension to the right ventricle it is not uncommon for arterial hypotension and bradycardia to appear, apparently mediated by a cardioinhibitory reflex (Bezold-Jarisch).29 This cardioinhibitory reflex has been observed following reperfusion of the right coronary artery by angioplasty30 or thrombolysis.31
The presence and severity of the physical signs of ARVI will depend on the degree of dysfunction and dilatation of the right ventricle; extensive infarction is characterized by the appearance of signs of severe right heart failure, such as hepatomegaly, pulsatile liver, ascites, and peripheral edema. These signs are not usually observed in the acute phase of infarction and their appearance 2 or 3 weeks later indicates extensive necrosis of the right ventricular myocardium extending to the right atrial myocardium.
ELECTROCARDIOGRAPHY
Right Ventricular Infarction
ST-segment elevation of more than 1 mm in V3R and V4R is very useful for establishing diagnosis of ARVI. Elevation of the ST segment in V4R was a very specific sign of right ventricular infarction in 18 cases with postmortem confirmation; survivors with that ECG abnormality had a higher incidence of hypotension and right heart failure than patients with inferior infarction restricted to the left ventricle.32
Elevation of the ST segment by more than 0.5 mm in the V4R lead is indicative of ARVI, with a sensitivity of 83% and a specificity of 77%33; those percentages increase when the ST-segment elevation is greater than 1 mm. Elevation of the ST segment in V3R and V4R can be transient and only registered in the first 24 or 48 hours following onset of infarction.34
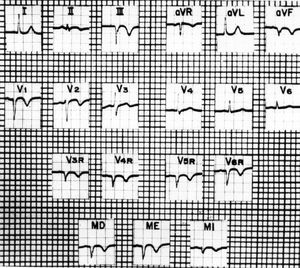
Medrano and de Micheli35-37 performed studies in dogs and humans to assess the usefulness of the precordial leads and upper abdominal unipolar leads known as MD (right hypochondriac region), ME (midepigastric), and MI (left hypochondriac region) in the presence of right ventricular myocardial infarction (Figure 3). When chemical necrosis (phenol) was produced in the posterior wall of the right ventricle in dogs, changes were recorded in the epicardial and thoracic leads associated with abolition of electromotive force originating in that region. In the DII, DIII, aVF, and V3R to V5R leads, along with the upper abdominal leads MD and ME, the ECG waveforms found were QS, Qr, or rS instead of RS, Rs, or R.
Figure 3. Electrocardiogram with right precordial and upper abdominal unipolar leads in the right hypochondriac region (MD), epigastric region (ME), and left hypochondriac region (MI), revealing signs of a transmural dead zone in the diaphragmatic surface of the left ventricle: QS complexes in aVF and DIII. Indicates invasion of posterolateral regions of the right ventricle: QS complexes in the right precordial leads from V6R to V3R, and in the upper abdominal leads MD and ME. Primary negative T waves in aVF, DII, DIII, V6R to V2, and in the upper abdominal leads, appear to be due to extensive subepicardial or transmural ischemia that covers the left posteroinferior and right posterolateral regions. Taken from Arch Inst Cardiol Mex. 1989;59:195-210.
Medrano and de Micheli38 also performed experimental studies on infarction of the anterior free wall of the right ventricle and found a reduction in the voltage of the R wave, or QS complexes, in the right epicardial and thoracic leads from V5R to V2 and V3, with signs of lesion (ST-segment elevation), and epicardial ischemia (negative T wave).
The development of disorders of intraventricular conduction such as right bundle branch block, or disorders of the left subdivisions has also been studied with ECG and vectorcardiogram recordings after producing chemical necrosis in the anterior portions of the interventricular septum, and the free wall of the right ventricle.39,40
In terms of electrocardiographic diagnosis of posterior infarction spread to the right ventricle in humans, in most cases the signs of necrosis are recognized in DIII, aVF, and from V3R or V4R to V6R, as well as in the MD and ME leads. QR complexes with a slurred R wave are recorded in cases with proximal or distal right block (Q waves of 35 to 60 ms).41 Medrano and de Micheli42,43 have provided very useful descriptions of the electrocardiographic manifestations of biventricular infarctions. Posterior biventricular infarctions are more common than anterior ones.
Signs of a dead zone in the free wall of the right ventricle are observed more often in posterior biventricular infarctions than in anterior ones. In these cases, the voltage of the ST-segment elevation is higher in the right thoracic leads than in V2 and V3, suggesting invasion of the anterior surface of the right ventricle.
Right Atrial Infarction
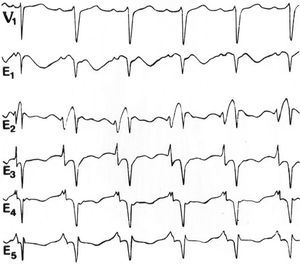
There has been interest in identifying the ECG abnormalities associated with atrial infarction, especially in the P wave and the atrial ST-T segment, for more than 65 years.10 The studies achieved greater validity in later years with the use of unipolar leads in the precordial region and the limbs.44 In studies performed by Medrano and de Micheli,45-47 in which right atrial infarction was generated in dogs by subepicardial injection of alcohol, the most significant changes were observed in the right thoracic leads, and sometimes up to V4-V5. The vector for the lesion points forward, elevating the P-R segment in the mentioned leads and in the direct right atrial leads. Qp or complex waveforms were recorded in W. In isolated cases with suspicion of right atrial ischemia, intraesophageal recordings have been used to detect ECG changes in the P wave and in the atrial ST segment48 (Figure 4).
Figure 4. Recordings with 5 intraesophageal leads, from E1 (the most cranial) to E5 (the most caudal), obtained simultaneously to recordings from a precordial V1 lead in a man aged 43 years. Note the elevation of the atrial ST segment in the E1 and E2 leads, indicating a right atrial lesion. The recordings were obtained at a speed of 50 mm/s; 1 cm=10 mm. Taken from Arch Inst Cardiol Mex. 1992;62:277-88.
Regarding the presence of ventricular arrhythmias, some studies have reported an increased incidence in patients with biventricular inferior infarction.49,50 There is also evidence that ventricular arrhythmias are more common in patients in whom attempted biventricular reperfusion of the myocardium was unsuccessful.51 The development of supraventricular arrhythmias, including atrial fibrillation, is more common in patients with ischemic right ventricular dysfunction52; these arrhythmias may be related to atrial ischemia or infarction, with distension of the atrial chamber, or with an increase in right atrial pressure.
ECHOCARDIOGRAPHY
Right Ventricular Infarction
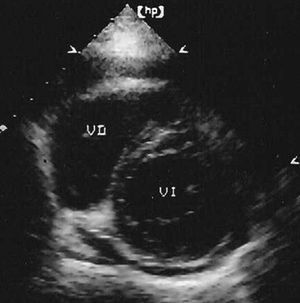
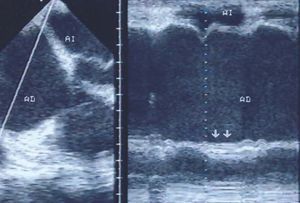
Echocardiography is useful to reveal the spread of infarction to the myocardium of the right ventricle. Parasternal images in the short axis and apical 4-chamber views provide the most information. The first shows the right ventricle with a crescent-moon morphology (Figure 5), while in the second, the chamber of the right ventricle tends to be triangular under normal conditions. These images can be used to determine the diameters of the right ventricular cavity as well as the motion of the anterolateral and inferior walls, and of the interventricular septum. Motion of the inferior and lateral walls of the right ventricle can also be assessed in subcostal images.
Figure 5. Parasternal echocardiogram in the short axis. The right ventricle appears dilated, with a crescent-moon morphology. Real-time images revealed reduced motion and/or a lack of systolic thickening in the compromised walls.
In patients with extensive right ventricular infarction and hemodynamic deterioration, the images show dilatation of the chamber and defective ventricular wall motion, commonly akinesia of the posteroinferior wall. Defects in the motion of the interventricular septum can be in systole (paradoxical motion) or in diastole. Defects in diastole are indicative of increased right ventricular pressure with inversion of the transseptal pressure gradient.53
Echocardiographic demonstration of wall dyskinesia may be more sensitive than hemodynamic abnormalities for the identification of right ventricular infarction. However, wall motion abnormalities have a low specificity, since segmental dyskinesia caused by ischemia can be present without myocardial infarction. The use of echocardiography has confirmed that the extent of right ventricular infarction is highly variable, ranging from a small hypokinetic region to marked dilatation of the chamber with a large area of wall dyskinesia.
In theory, the hemodynamic consequences of right ventricular infarction could be recognized by calculation of the ventricular volumes and the ejection fraction. However, this has not been possible since the complex morphology of the ventricle has limited the use of geometric formulas to calculate its volume. Nevertheless, some echocardiographic parameters have been described that are correlated with the right ventricular ejection fraction obtained by radionuclide ventriculography. These echocardiography parameters include the ratio between the end-diastolic size of the 2 ventricles, descent of the right ventricular base, and inspiratory collapse of the inferior vena cava.54,55
Apical and subcostal images can show leftward displacement of the interatrial septum, which is a sign found in very extensive right ventricular infarctions or when ischemia extends to the walls of the right atrium. This echocardiographic sign is an indicator of poor prognosis, since it is often associated with arterial hypotension, advanced atrioventricular block, and increased mortality.56
The right ventricular ejection fraction has been quantified in patients with inferior AMI, with or without extension to the right ventricle. The formula used was based on area-length of a pyramid, taking the ejection fraction (equilibrium radionuclide angiography) as a reference. The sensitivity and specificity obtained for ejection fractions less than 30% were 69% and 97%, respectively.57
Echocardiographic assessment of myocardial reperfusion with contrast agents injected in the peripheral veins or coronary arteries following angioplasty, or thrombolysis has mainly focused on the left ventricle. A few reports have indicated the usefulness of this technique to assess myocardial perfusion and contractile reserve (dobutamine) of the right ventricle following postinfarction coronary angioplasty.58
In patients with AMI of the right ventricle, intravenous infusion of low doses of dobutamine (5-10 µg/kg/min) has been used to assess myocardial viability following reperfusion therapy. Significant improvement of overall right ventricular function following dobutamine indicates recovery of a stunned myocardium. The echocardiographic criteria for right ventricular myocardial viability are as follows: demonstration of systolic thickening of the wall in a previously akinetic segment or recovery of wall thickening in a previously hypokinetic segment.59
Doppler Echocardiography
In the presence of right ventricular infarction, the use of Doppler to assess blood flow in the right chambers offers information related to hemodynamic abnormalities. Spectral analysis of blood flow during right ventricular filling usually reveals 2 peaks: the early diastolic or rapid filling (E wave) and the end-diastolic following atrial contraction (A wave). When the ischemia includes the right atrium, its mechanical activity is diminished or can even disappear (extensive atrial necrosis); this is translated into a reduction or absence of the A wave. It is important to note that there are other conditions in which Doppler analysis of right ventricular filling flow only shows an E wave, as is the case in patients with atrial fibrillation and in some disorders of atrioventricular conduction.
It has been reported that in the presence of very extensive right ventricular infarction, Doppler analysis can reveal that ventricular filling and ejection flows are very slow and last almost the entire cardiac cycle. This flow pattern, similar to that of a vein, indicates that the right ventricle has lost pumping function and behaves as a passive conduit.60
In the large majority of patients with right ventricular infarction, Doppler imaging reveals tricuspid regurgitation, which is associated with dysfunction of the subvalvular apparatus and, occasionally, annular dilatation. It has been shown that Doppler imaging in patients with inferior infarction of the left ventricle and pulmonary valvular regurgitation can allow identification of those patients in whom infarction has spread to the right ventricle.61
Overall systolic and diastolic ventricular function can be assessed using the Tei index in Doppler echocardiography. This index is calculated as the sum of the isovolumetric ventricular contraction and relaxation times divided by the ejection time. The various elements of the formula can be quantified through Doppler recordings of right ventricular filling and ejection flows.
The normal value of the Tei or myocardial performance index for the right ventricle is 0.25 (0.05); in patients with inferior infarction of the left ventricle, a Tei index of more than 0.30 indicates extension of the infarction to the right ventricle, with a sensitivity of 82% and a specificity of 95%.62 The usefulness of this index is abolished in the presence of pulmonary heart disease, arrhythmias, or severe left ventricular dysfunction. Likewise, the Tei index, which is generally increased in patients with infarction of the right ventricular myocardium, can be pseudo normalized when the infarction is very extensive and the right atrial pressure is greater than 15 mm Hg. In these patients with severe ventricular dysfunction, the isovolumetric contraction time may be normal, or still very short, due to equalization of the diastolic pressure in the right ventricle and the pulmonary artery.63
Ischemic diastolic dysfunction of the infarcted right ventricle reduces myocardial compliance, with an increase in the right ventricular and atrial end-diastolic pressures. Under those conditions, when there is also a patent foramen ovale, the right atrial pressure is greater then the left atrial pressure and right-to-left arteriovenous shunt is developed. The appearance of cyanosis and refractory hypoxemia in a patient with recent right ventricular infarction indicates that interatrial shunt must be considered.
This complication can be confirmed with Doppler echocardiography, preferably with transesophageal recordings. This technique also allows differentiation between patent foramen ovale and a true septal defect, helps to elect the most appropriate treatment, and also provides information on the contractility of the right atrium. Laham et al64 reported a case series of 5 patients with interatrial shunt and infarction of the right ventricular myocardium, associated with inferior left ventricular infarction in 4 patients and isolated right ventricular infarction in 1. Patent foramen ovale was confirmed in all 5 patients and in 2 patients right-to-left shunt was manifested by a cerebral ischemic event as a result of paradoxical embolism. This interatrial shunt can be treated pharmacologically,65 through the use of a percutaneous closure device, or surgically.64
Tissue Doppler
Tissue Doppler echocardiography quantifies myocardial velocities and has been used to assess overall ventricular function. Assessment of the free wall of the right ventricle can be performed using an apical 4-chamber view; it is recommended that the myocardium being studied is located 1 cm from the tricuspid ring, oriented towards the ventricular apex. With tissue Doppler, the systolic myocardial velocity is recorded as a positive curve following valve displacement towards the ventricular apex; in diastole, when the tricuspid valve is displaced from the apex towards the base, 2 negative early diastolic and end-diastolic curves are recorded.
In healthy subjects, the values for tricuspid annular velocity in systole, early systole, and end-systole are around 14.5, 14, and 16.5 cm/s, respectively; in patients with inferior infarction extending to the right ventricle, the values were 10.3, 8.2, and 13.6 cm/s, respectively (P<.001).66 In patients with inferior infarction extending to the right ventricle, other authors have recorded even lower tricuspid annular velocities using tissue Doppler, especially for the systolic and early diastolic velocities.67,68
In a recent study, Dokainish et al69 confirmed that the systolic and early diastolic myocardial velocities for the free right ventricular wall allow extension to the right ventricle to be identified in patients with inferior infarction. In addition, they showed that systolic myocardial velocity less then 8 cm/s can predict adverse events (hospitalization or death) at 1-year follow-up with a sensitivity of 85% and a specificity of 77% (area below the curve= 0.82; P<.001). This parameter obtained from tissue Doppler is an independent predictor of outcome associated with proximal obstruction of the right coronary artery.69
Tissue Doppler and recording of myocardial velocity at the tricuspid annulus allows measurement of the systolic and diastolic intervals. In this way, the myocardial performance or Tei index (described earlier) can be determined, allowing assessment of overall ventricular function. In patients with inferior infarction extended to the right ventricle and proximal obstruction of the right coronary artery, the values of the Tei index are greater than 0.70.70
Transesophageal Echocardiography
In patients with right ventricular myocardial infarction, transesophageal echocardiography is useful when there are symptoms of severe hemodynamic deterioration, suspicion of ischemia or infarction of the right atrium, or an inadequate transthoracic acoustic window. The examination is performed in the coronary care unit with the patient lightly sedated. Continuous ECG recording and monitoring of blood pressure and peripheral oxygen saturation is employed and the procedure lasts around 15 to 20 minutes.
The echocardiogram must include both transesophageal and transgastric recordings. The transesophageal recordings should be used to assess segmental wall motion of both ventricles, for which a 4-chamber view should be used, along with the long axis of each ventricle. These images can be used to visualize the various ventricular walls, except the apical segments of the right ventricle.
Transesophageal examination should also include analysis of right atrial wall motion, along with anatomic and functional characteristics of the atrioventricular valves. The examination is complemented by Doppler analysis of filling flow in both ventricles and color imaging is used to assess the presence of interatrial shunt or tricuspid and mitral regurgitation.
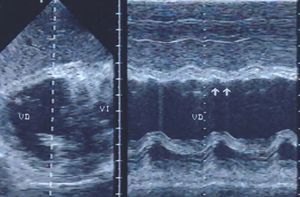
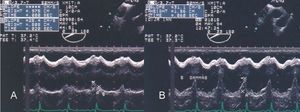
Assessment of ventricular wall motion is completed with transgastric images in various planes (Figure 6). The apical segments of the right ventricle can be selectively visualized through the use of transgastric images at 110o to 130o. Transgastric images also provide comprehensive information on the condition of the mitral subvalvular apparatus.
Figure 6. Transgastric echocardiography in a patient with posteroinferior infarction of the left ventricle extending to the right ventricle. Real-time and M-mode recordings revealed akinesia in the posterior wall of the right ventricle (arrows).
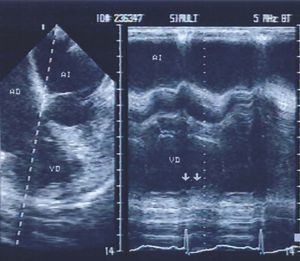
In the first case series of acute inferior wall infarction of both ventricles in which we employed echocardiography, we found that in all 11 patients studied, transesophageal and transgastric recordings revealed areas of right ventricular dyskinesia (Figure 7), while this was only apparent in 6 patients when using transthoracic recording.71 In addition, in 2 patients, only transesophageal echocardiography revealed ischemic involvement of the right atrium.
Figure 7. Four-chamber transesophageal echocardiography in a patient with posteroinferior infarction of the left ventricle extending to the right ventricular walls. Real-time and M-mode recordings revealed dyskinesia of the right ventricular wall (arrows).
In another study we used transesophageal echocardiography to assess 38 patients with AMI spread to the right ventricle and determined the wall motion index for both ventricles. In all 38 patients, abnormalities were observed in posteroinferior wall motion, and in some patients the dyskinesia extended to other regions of the ventricles. Comparison of in-hospital progress and progress following hospital discharge (follow-up, 6-60 months) with the wall motion index for both ventricles revealed that the 6 patients with New York Heart Association (NYHA) functional class III-IV had the worst motion indexes and 4 of those patients died. In contrast, of the 32 patients in NYHA functional class I-II, 26 (81%) had a better motion index and there were no deaths recorded in that subset of patients. These findings indicate that in the presence of biventricular infarction, the wall motion index obtained by transesophageal echocardiography is related to the extent of myocardial damage, in-hospital mortality, and functional class at hospital discharge.72
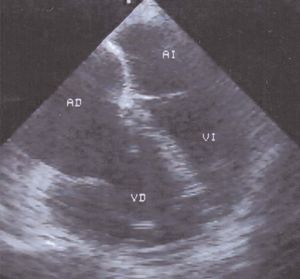
Likewise, the simple ratio of ventricular diameters (right ventricle/left ventricle), obtained in the transesophageal 4-chamber view identifies those patients with the greatest hemodynamic repercussions of right ventricular infarction (Figure 8). In 30 patients with a right to left ratio of 1 or less, the hemodynamic consequences of the infarction were mild and in the period following hospital discharge they maintained a functional class of I to II. The right to left ratio was greater than 1 in the remaining 8 patients; 6 of those patients were in functional class III-IV and 4 died.72
Figure 8. Four-chamber transesophageal echocardiography in a patient with posteroinferior infarction of the left ventricle extending to the right ventricle. The image reveals dilatation of the right chambers and the interatrial septum is displaced towards the left. The right ventricle is dilated and its dimensions are larger than those of the left ventricle.
Transesophageal echocardiography is not only of use in identifying right atrial and ventricular ischemia, but also allows assessments of various treatments used in the early phase of infarction and helps to differentiate irreversible myocardial damage from myocardial stunning.
Based on evidence that echocardiography with dobutamine can reveal reversible postischemic myocardial dysfunction, we studied 9 patients with inferior AMI extending to the right ventricle. In an effort to identify viable myocardium following ischemia, transesophageal echocardiography was performed with infusion of low doses of dobutamine (5 and 10 µg/kg/min) and the results were compared with those obtained through nuclear imaging of myocardial perfusion (sestamibi-SPECT). Based on changes in wall motion and systolic thickening produced by dobutamine, echocardiography yielded information comparable to that obtained by nuclear imaging of myocardial perfusion, specifically in the identification of myocardial viability in both ventricles following myocardial infarction.73
Right Atrial Infarction
Right atrial infarction is difficult to diagnose with conventional transthoracic echocardiography, due to the difficulty of assessing atrial wall motion. Consistent with our experience, in recent years transesophageal echocardiography has become the diagnostic method of choice to confirm the presence of right atrial infarction.
Transesophageal images (Figure 9) allow diagnosis of right atrial myocardial infarction to be established according to the following criteria: a) akinesia of the right atrial wall, in the presence of left atrial contraction; b) right atrial spontaneous contrast; c) thrombosis at the site of atrial wall akinesia; d) absence of an A wave in the curve for tricuspid flow, with an A wave present in the mitral flow.74 When the mentioned signs are detected in a patient with ventricular myocardial infarction, diagnosis of right atrial ischemia or infarction is valid.
Figure 9. Four-chamber transesophageal echocardiography in a patient with posteroinferior infarction of the left ventricle extending to the right ventricle and right atrium. Directed M-mode recordings reveal akinesia of the right atrial wall (arrows). There is a spontaneous contrast effect in the chamber along with a small pericardial effusion.
It is important to mention that, as occurs with the ventricular myocardium, echocardiographic evidence of hypokinesia, and akinesia is not necessarily indicative of myocardial ischemia or necrosis. We have observed absence of wall motion for both atria in patients with evolving myocardial infarction and electrocardiographic evidence of sinus arrest, while administration of atropine restored sinus rhythm and normalized atrial wall motion. Other patients with atrioventricular conduction disorders may have wall motion abnormalities without atrial infarction. With transesophageal echocardiography it is possible to study normal contraction of the atrial wall in detail; this is characterized by thickening of the myocardium and displacement of the endocardium towards the center of the chamber (Figure 10A). Pharmacologic stimulation of the atrial myocardium with dobutamine also increases wall contraction (Figure 10B).
Figure 10. A: transesophageal echocardiography at an angle of 30o, oriented to visualize the interatrial septum and the right atrial wall. Study performed in a subject with a normal heart. The image shows a normal contraction amplitude for the atrial wall (A wave). B: normal response to stimulation with intravenous dobutamine at a dose of 5 µg/kg. Extensive motion of the right atrial wall is observed.
In patients with posterior infarction of the right ventricle or of both ventricles, if transesophageal echocardiography reveals right atrial motion abnormalities, the source of the wall dyskinesia must be clarified. The abnormal contraction may be a result of transient ischemia in a stunned atrial myocardium or it could be the expression of infarction without viable myocardium; these possibilities can be differentiated through the use of echocardiography and pharmacologic stimulation with dobutamine. Increased atrial motion with dobutamine in a region that was previously hypokinetic is indicative of myocardial stunning. In those cases, coronary angiography shows that right atrial circulation is not completely blocked. In contrast, in the presence of atrial necrosis, wall akinesia is not altered with dobutamine and coronary angiography does not reveal right atrial branches.75
The usefulness of transesophageal echocardiography to demonstrate right atrial ischemia and necrosis, as well as the positive inotropic effect of dobutamine on the right atrial myocardium, has also been demonstrated in canine experimental models.76
Transesophageal echocardiography is useful to assess the prognostic implications of reperfusion therapy (thrombolysis and/or coronary angioplasty) in patients with inferior wall infarctions of both ventricles, with or without extension of ischemia to the right atrium.77 In that study, it was demonstrated that echocardiographic evidence of right atrial ischemia is associated with higher indices of abnormality in right atrial motion, dilatation of the right ventricular chamber, and proximal obstruction of the right coronary artery, as well as a higher incidence of arrhythmias, atrioventricular block, and death. Rapid treatment with thrombolysis or angioplasty is associated with less alteration of wall motion and less dilatation of the right ventricle.77
NUCLEAR CARDIOLOGY
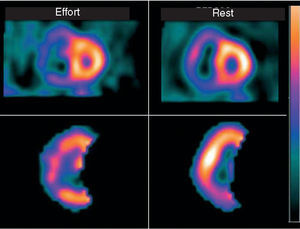
Scintigraphic imaging of myocardial perfusion with single-photon emission computed tomography (SPECT) or ECG-gated SPECT is very useful for the diagnosis of ischemia or AMI, in risk stratification and assessment of prognosis, and in recognizing spread of myocardial ischemia to the right ventricle (Figure 11).
Figure 11. Nuclear cardiology study using single-photon emission computed tomography labeled with sestamibi (sestamibi-SPECT) in a patient who presented a small left ventricular infarction extending to the right ventricle. Marked dilatation of the right ventricle is observed both at rest and with effort. At rest there is hypoperfusion of the inferior wall, while on effort the region of hypoperfusion extends towards the lateral wall, indicating ischemia at this site.
Protocols using a variety of radioactive tracers have been designed for use with these techniques. The most widely used are 99mTc-sestamibi at rest, complemented with exercise or pharmacologic stress, or a dual protocol, which includes thallium-201 at rest and 99mTc-sestamibi in the pharmacologic phase or during exercise.
SPECT allows selective assessment of the right ventricle, which increases the myocardial concentration of the radiolabeled drug and facilitates analysis of perfusion and motion of the anterior, lateral, and inferior walls, as well as the inferolateral and anterolateral segments. The amount of at-risk myocardium and the viable segments of the ventricular wall can be identified. The use of gated-SPECT, in addition to revealing abnormalities in regional or generalized motion, also allows demonstration of systolic thickening.
Quantification of end-diastolic and end-systolic volumes, and right ventricular ejection fraction using nuclear cardiologic techniques offers advantages over other techniques such as echocardiography, since it does not depend on ventricular geometry and is based on the amount and density of the radionuclide. The use of first-pass or equilibrium techniques has shown that 40% to 50% of patients with inferior left ventricular infarction have ischemic right ventricular dysfunction.78,79 Factors other than an ischemic process in the right ventricle can lead to a reduction in its ejection fraction, among them chronic obstructive pulmonary disease, pulmonary embolism, and valvular lesions with pulmonary hypotension.
In a prospective study, radionuclide ventriculography allowed identification of hemodynamically significant right ventricular myocardial infarction based on an ejection fraction of less than 40%, associated with segmental wall motion abnormalities (akinesia or dyskinesia); the sensitivity was 92% and the specificity 82%.24 Table 1 compares these results with the results obtained with other diagnostic techniques.
Another method employed in the diagnosis of right ventricular myocardial infarction is dual SPECT with 201Tl and 99Tc. With this technique used at 2 to 9 days following onset of infarction, a lower prevalence of right ventricular ischemia was found in patients with early thrombolytic therapy compared with those who did not receive thrombolytic treatment (26.7% vs 68.4%, P<.01).80
In another group of 30 patients with inferior infarction, with the use of 201Tl and 111I antimyosin the presence of indium antimyosin was demonstrated in 14 patients (47%); in 13 of those patients perfusion defects were also revealed with 201Tl.81 Finally, in 33 patients with inferior infarction, the use of sestamibi-SPECT with low-grade physical exercise, applied 6 to 14 days after myocardial infarction, allowed detection of right ventricular perfusion defects in 10 cases (30%).82 Half of those reperfusion defects were absent or reduced at rest.82
MAGNETIC RESONANCE IMAGING
Assessment of the right ventricle can be undertaken with the patient at rest, during exercise, or with pharmacologic stimulation. Real-time studies require ECG synchronization. Assessment of ventricular anatomy can be undertaken with the sequential spin-echo technique, while functional assessment is performed with cine magnetic resonance (gradient-echo). Assessment of systolic function has been preferentially undertaken with images in the short axis or transverse plane--it should be remembered that the true short axis of the right ventricle has a different orientation to that of the left.
Various protocols for assessment of the right ventricle include image acquisition in the short and long axes of the ventricles, as well as in the 4-chamber view. Diagnostic analysis of the images should include the use of Simpson's rule with manual or semiautomated planimetry of the endocardial borders of each ventricle during end-diastole and end-systole, as well as the epicardial border in end-diastole. This allows quantification of ventricular volumes and it is possible to calculate stroke volume, ejection fraction, and right ventricular mass.83,84
Assessment of systolic function for each segment of the right ventricular wall can be performed with magnetic resonance imaging (cardiac MRI tagging). With this technique it is possible to quantify the percentage shortening of the segment and vector analysis allows the trajectory of the free ventricular wall to be determined during systole. It has been demonstrated that the free ventricular wall moves towards the ventricular outflow tract and the interventricular septum, and the percentage shortening is greater in the apical segments, and lesser in the basal segments.85 Two-dimensional reconstruction obtained using this technique revealed that the right ventricle, in addition to its circumferential contraction, has a slight gyratory movement.86
MRI analysis of myocardial perfusion employs the same concept used in nuclear cardiology, contrast echocardiography, and x-ray densitometry, which involve administration of a tracer and detection of its movement, and distribution through the heart. While techniques that involve injection of an exogenous agent are the most widely employed, there are others that involve the use of endogenous contrast.
First-pass MRI offers spatial and temporal resolution of myocardial perfusion, making it the noninvasive method of choice. As the spatial resolution of MRI is 2 mm or less, it is possible to identify ischemia limited to the subendocardium, a region with greater susceptibility to ischemia and necrosis.
Contrast MRI with late enhancement is useful not only for identification of viable myocardium but also in determining whether myocardial ischemia is transmural or subendocardial. However, the results are not specific to myocardial infarction, since similar images are obtained in inflammatory processes of the heart such as sarcoidosis or endomyocardial fibrosis, and also in hypertrophic cardiomyopathy. The difference is that in these nonischemic myocardial abnormalities the late enhancement detected in the MRI does not affect the subendocardium.87
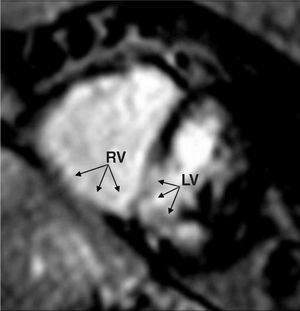
Diagnosis of right ventricular myocardial infarction with MRI and gadolinium can be established with the same sensitivity and specificity as with the use of a dual-SPECT nuclear cardiology protocol and even with less time required for image acquisition88,89 (Figure 12).
Figure 12. Magnetic resonance imaging in a patient with posterobasal transmural infarction of the left ventricle extending to the basal segments of the right ventricle (arrows). Dilatation of the right ventricle is seen along with hypoperfusion of the inferior wall of both ventricles and the posteroinferior portion of the interventricular septum. RV indicates right ventricle; LV, left ventricle.
In patients with right ventricular infarction that was not recent, MRI has been shown to be useful to quantify volumes and right ventricular ejection fraction, and the results obtained have been similar to those generated with thermodilution using Swan-Ganz catheters.90
In patients with inferior infarction of the left ventricle, spin-echo MRI reveals changes secondary to postinfarction remodeling. As a result of ventricular interdependence, those changes also affect the right ventricle (diastolic dysfunction) and the right atrium (increased volumes and reduced ejection fraction). Reverse remodeling can also be visualized with MRI following successful myocardial reperfusion therapy.91,92 The late phase of right ventricular remodeling, in other words, the development of postinfarction aneurysm, has also been diagnosed with MRI.93
PROGNOSIS
Patient progress following right ventricular infarction is related to the extent of left ventricular necrosis and early mortality may be associated with mechanical or electrical complications of left ventricular dysfunction.94 However, it has also been reported that many patients died from right ventricular infarction with serious hemodynamic consequences and in whom both left coronary circulation and left ventricular systolic function were normal.
Some studies have demonstrated the importance of pressure curves for the right atrium in assessing the initial prognosis of right ventricular infarction.27 While the presence of prominent A waves has been associated with improved prognosis, when the A wave is reduced, the prognosis is poor and coronary angiography reveals proximal obstruction of the right coronary artery or circumflex artery that compromise myocardial blood flow in the right atrium.
According to our studies using echocardiography, prominent A waves in the right atrium are caused by hyperkinesia of the free atrial wall. This increase in mechanical activity of the atrium attempts to compensate for the ischemic systolic and diastolic dysfunction of the right ventricle. In contrast, reduced or absent A waves are caused by a lack of atrial mechanical activity, as a result of right atrial ischemia or infarction.75
Patients with inferior infarction of the left ventricle and extension of the ischemic process to the right ventricle and right atrium have a poor prognosis, since hospital stay is longer and mortality higher, and there is a greater likelihood of supraventricular and ventricular arrhythmias, and complete atrioventricular block.77
Medium-term and long-term prognosis is generally good when inferior infarction is limited to portions of the right ventricle and the period of hospital stay passes without serious complications. In another small group of patients, spread of ischemia to the right ventricle leads to greater morbidity and mortality due to ventricular dysfunction, and reduction of cardiac output. Thrombolytic therapy or primary coronary angioplasty reduce complications during hospital stay and lead to an excellent long-term prognosis in those patients. This improved prognosis is independent of the type of myocardial reperfusion used and the extent of myocardial infarction in the right ventricle.95
Patients with cardiogenic shock as a result of myocardial infarction predominantly of the right ventricle have a high rate of in-hospital mortality (53.1%), similar to that of patients with myocardial infarction predominantly of the left ventricle (60.8%).96 In this group of patients with cardiogenic shock it has been observed that, once the period of hospital stay has passed, the presence of right ventricular dilatation (left-to-right end-diastolic ventricular ratio <2) identifies a subset of patients with inferior infarction, usually with single-vessel obstruction, and with greater survival at 1-year follow-up (70% vs 34%).97
In a study of 302 patients with AMI of the right ventricle, divided into 3 groups according to the presence of right ventricular dysfunction or cardiogenic shock (36 patients), it was found that in the group with cardiogenic shock, primary coronary angioplasty reduced mortality from 89.5% to 58%.98
TREATMENT
Treatment is based on 4 elements: a) maintenance of preload; b) reduction of afterload; c) inotropic support of the dysfunctional right ventricle; and d) early revascularization (Table 2). Treatment of the patient with myocardial infarction extending to the right ventricle has included volume loading by infusion of liquids, but this treatment should be restricted to patients with low intravascular volume. Administration of 300 to 600 mL of saline solution in a 10 to 15 minute period can increase blood pressure and cardiac index. Invasive monitoring (Swan-Ganz catheter) or noninvasive monitoring (Doppler echocardiography) of the chamber pressures and pulmonary pressure, as well as cardiac output, helps to appropriately assess the need for parenteral fluids.
Treatment with inotropic drugs has preferentially included dobutamine, which maintains preload and increases systolic function, leading to an increase in right ventricular motion and stroke volume. It should be remembered that, by increasing heart rate and contractility, excessive doses of inotropics can cause ischemia as a result of an increase in the difference between supply and demand of oxygen in the myocardium. Dopamine, which has a greater vasoconstrictive effect, can be used in some patients with severe hemodynamic deterioration.
Myocardial reperfusion therapy with thrombolytic agents or coronary angioplasty in patients with right ventricular infarction has been shown to reduce the rate of complications in the initial period of hospitalization and also to reduce mortality (Table 2). Studies published on this treatment have included patients with obstruction of various coronary arteries, as well as previous infarction of other myocardial walls. Consequently, the potential benefit of reperfusion of the causative artery can not be determined.
Our group designed a study to assess the short-term and long-term effects of early reperfusion in 122 patients with obstruction of the right coronary artery in isolation and inferior myocardial infarction with or without extension to the right ventricle (52 and 70 patients, respectively). All patients received reperfusion therapy with primary coronary angioplasty or thrombolysis followed by elective angioplasty. In 16 of the 122 patients (8%; 7 without and 9 with right ventricular infarction) persistent occlusion of the causative artery was detected. Comparison of that subset of patients with those patients who had a patent artery revealed a higher incidence of ventricular and supraventricular arrhythmias, and of atrioventricular block. During clinical follow-up of 8 to 36 months, the progress of the 118 survivors was satisfactory, except in 1 patient with right ventricular infarction and rupture of the interventricular septum who died 1 year later; the remaining 117 patients were in functional class I.95 According to these results, in patients with inferior myocardial infarction with and without right ventricular involvement, acute reperfusion with thrombolysis, or primary angioplasty is the treatment of choice to reduce complications during the period of hospital stay and improve medium-term and long-term prognosis.
Patients with right ventricular infarction often present atrioventricular conduction disorders and/or persistent arterial hypotension. Treatment with a sequential pacemaker has a beneficial effect on atrial contraction by increasing ventricular filling and stroke volume.99 It is very likely in this type of patients that the ischemic process has spread to the right atrial myocardium.
Treatment of cardiogenic shock due to right ventricular infarction with dobutamine and vasodilators can have effects that are limited by the presence of arrhythmias, systemic vasodilation, and arterial hypotension. Patent foramen ovale can cause hypoxemia secondary to right-to-left atrial shunt. This hypoxemia is resistant to conventional treatment with supplementary oxygen.
In contrast, reduction of insufficient right ventricular afterload with a selective pulmonary vasodilator can improve cardiac function without systemic vasodilation and arterial hypotension.
Inhalation of nitric oxide causes relaxation of smooth muscle cells and this reduction in pulmonary vascular tone has been observed in patients with various forms of pulmonary hypertension (primary or secondary). Likewise, a reduction in afterload on inhalation of nitric oxide has been demonstrated in an experimental swine model of right ventricular infarction.100
In a recent study undertaken in 13 patients with right ventricular myocardial infarction and cardiogenic shock it was found that treatment with inhaled nitric oxide reduces the mean pressure in the right atrium, the mean pulmonary arterial pressure, and the pulmonary vascular resistance, as well as increasing the cardiac index and the stroke volume. In that study it was confirmed that there were no effects of nitric oxide on pulmonary capillary and systemic arterial pressure. Another effect of nitric oxide was a reduction in arteriovenous shunt via the foramen ovale.101 Those results demonstrate a beneficial effect of inhaled nitric oxide in the treatment of cardiogenic shock due to right ventricular infarction.
In the presence of treatment resistant severe right ventricular dysfunction following myocardial infarction, placement of an intraaortic balloon pump should be considered, or even better, use of a right ventricular mechanical support system. This device can improve deteriorated right ventricular function and can serve as an interim treatment (72-96 hours) prior to surgical correction of coronary artery obstruction.101
The use of percutaneous septostomy of the interatrial septum has been proposed to improve preload and left ventricular function, as well as decompress the right ventricle, in patients with refractory cardiogenic shock following right ventricular infarction. Transseptal puncture of the atrial septum (Brockenbrough needle) can be facilitated through the use of transesophageal echocardiography; the aim of the procedure is to create a wide interatrial conduit through a right-to-left atrial shunt. In studies of isolated cases, this procedure has been found to increase systolic blood pressure and cardiac index as a result of increased left ventricular filling. Likewise, echocardiography has shown functional recovery of the right ventricle and the absence of residual interatrial shunt.102
Correspondence: Dr. J. Vargas-Barrón.
Departamento de Ecocardiografía. Instituto Nacional de Cardiología Ignacio Chávez.
Juan Badiano, 1 Col. Sección XVI. 14080 Tlalpan. México DF. México.
E-mail: eco_vargas@terra.com.mx