Keywords
INTRODUCTION
In spite of ongoing advances in percutaneous revascularization, certain clinical and angiographic profiles are still associated with worse outcomes, both immediate and long term. The presence of an intracoronary thrombus allows identification of a subgroup of patients who are at greater risk for acute complications, infarct, and restenosis.1-7 The fact that primary angioplasty has proven to be the most effective treatment for acute myocardial infarction (AMI), along with the proven advantages of early revascularization in unstable coronary syndromes, has increased the number of thrombus-containing lesions treated in the hemodynamic laboratory.
Several improvements have been made in the treatment of lesions with a high thrombotic content. The stent, initially contra-indicated in the presence of intracoronary thrombus, has been shown to be beneficial in this type of lesion,8 although not as beneficial when an intracoronary thrombus is not present. As a contributory factor, thrombolysis has been associated with complications and adverse events.9 Platelet glycoprotein IIb/IIIa receptor antagonists, which have obvious benefit in angioplasty in patients with unstable coronary syndromes10,11 and AMI,12-14 have not been shown to have a positive effect in patients with intracoronary thrombus that is identifiable on angiogram.6,15
After the initial trials with thrombus extraction devices (AngioJet Possis Medical, Inc., Minneapolis, Minn.),16,17 new thrombectomy devices have been developed,18-25 along with distal protection systems26,27 in an attempt to eliminate the thrombus from the coronary tree and to avoid its distal embolization.
In this study, we present our experience in our laboratory with 2 new thrombectomy devices based on aspiration of intracoronary material in lesions with a high thrombotic content.
METHODS
Study design
The study was a prospective observational register of the results of using 2 coronary intervention devices at a single medical center.
Patients
We included in our analysis all the patients in whom a thrombectomy device was used in our center from June 1, 2000, to February 15, 2002.
Materials and methods
Both the use of a thrombectomy device and the type of device used, as well as the use of IIb/IIIa platelet receptor antagonists and other devices or treatments used during the procedure were according to the care giver´s instructions. All patients systematically received aspirin (200 mg per day) before the procedure (or 300 mg before the procedure if they had not previously been taking aspirin). All patients were anticoagulated with intravenous heparin sodium until an activated coagulation time (ACT) of more than 300 s was reached or, in patients in whom IIb/IIIa platelet antagonists were being used, between 200 and 250 s. The usual hemodynamic laboratory protocol consisted of capturing on film each device used during the procedure, in addition to an injection immediately following the use of each device. At the end of the procedure, filming of at least 2 orthogonal projections without intracoronary guide was performed, on which the final result of the treatment performed was visible.
DEFINITIONS
The use of thrombectomy devices was defined as the attempt (whether or not successful) to access the lesion for which said device was considered to be indicated. Rescue was defined as the indication for thrombectomy due to the occurrence of complications during the procedure, and elective to the rest of the indications. Direct was defined as the use of thrombectomy devices without prior dilatation or the use of another device. The presence of a thrombus on angiography was determined by the presence of a intraluminal filling defect outlined by contrast material, visible at least in 2 orthogonal projections, or by the presence, in totally occluded arteries, of a convex border with retention of contrast material.28-30 Coronary flow in the vessel treated was rated from 0 to 3, in accordance with the TIMI (Thrombolysis in Myocardial Infarction)31 qualitative scale, which was measured at: a) at the time of the diagnostic procedure; b) on the projection immediately before performing the thrombectomy; c) on the projection immediately following the thrombectomy, and d) at the end of the procedure. The result of the thrombectomy on angiographic imaging of the thrombus was subjectively quantified into 5 grades: 1, worsening on imaging; 0, no change on imaging; 1, slight reduction in the thrombus on imaging; 2, substantial reduction in the thrombus on imaging, and 3, complete disappearance of the thrombus on imaging. Distal embolization was defined as the visualization, at any point during the procedure, of a thrombus or amputation of a branch or fragment of a thrombus in a location distal to the lesion that was being treated. The ultimate angiographic success of the intervention was defined as the achievement of TIMI III final flow with visual stenosis of less than 20% without major complications.
Data collection
Clinical and procedural data were collected prospectively in an integrated manner from the existing interventionism registry in our laboratory. Analysis of angiographic filming was performed by 2 experienced interventionists off-line, via Phillips Inturis version 2.1 visualization software.
Devices
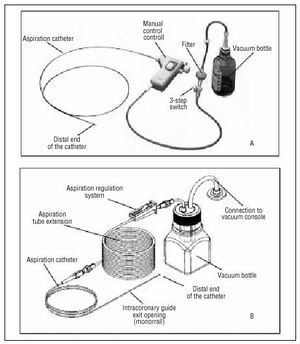
Two of the thrombectomy systems currently on the market were used. The X-Sizer device (EndiCOR Medical Inc., San Clemente, Calif., USA) has been described previously in this journal.24 It is a coaxial system used in the current study in its 2 forms, 2 mm (with a 5.5 Fr catheter diameter, compatible with 8 Fr catheters) and 1.5 mm (with a 4.5 Fr diameter, compatible with 7 Fr catheter diameters). Together with aspiration via it connection to a vacuum bottle, its mechanism of action is based on breaking up the thrombotic material via a helicoidal cutter located in the distal end of the catheter, controlled by a manual module operated by the interventionist during the procedure (Figure 1). The Rescue device (Boston Scientific Scimed, Inc. Maple Grove, Minn., USA) is a monorail system consisting of a catheter with 2 leads (Figure 1), one for passing the guide catheter, and the other through which aspiration of the intracoronary material is performed and is joined at the proximal end of the catheter, to an extension tube, which is in turn connected to a vacuum bottle. The bottle empties through another connecting tube that is connected, via a hydrophobe filter, to an aspiration console that increases the vacuum in the bottle. The aspiration rhythm is controlled by external compression of the catheter with a clamp. With both devices, the aspiration technique is based on the introduction of the catheter into the coronary artery and advancing it slowly until it reaches the site where the thrombus in located.
Fig. 1. A: X-Sizer (EndiCOR Medical Inc., San Clemente, CA, USA) coronary thrombectomy device). B: Rescue (Boston Scientific Scimed, Inc. Maple Grove, Minn., USA) coronary thrombectomy device.
Statistical analysis
Qualitative variables are expressed as absolute values and percentages, and compared by means of the Pearson χ² test. Quantitative variables are expressed as mean and standard deviation and were compared by means of the Student t test. The SPSS version 10.0 statistical package was used for the analysis.
RESULTS
One hundred and thirty-nine devices were used in 137 patients. In 1 patient in whom the lesion could not be accessed the X-Sizer device was used and was reached with the Rescue device. In another patient thrombectomy with the Rescue device of the circumflex artery was performed to extract a thrombus that was embolized from the descending anterior artery, where a thrombectomy had been performed previously.
Patient characteristics are shown in Table 1. The majority of the procedures performed were urgent (86% of patients). The primary indication for the procedure was an AMI (80% of patients), and in 96% of cases a primary angioplasty was performed. The use of angiography represented 22% of the total number of angioplasties performed in cases of AMI during the study period (n=508), reaching up to 41% usage during the last 6 months of the study (59 thrombectomies/113 angioplasties in AMI). We did not note significant differences in either the clinical characteristics or general procedural characteristics between those patients in whom a thrombectomy was performed as a first angioplasty device and those patients in whom thrombectomy was not performed, except in the lesser presence of previous infarct, the greater use of platelet IIb/IIIa receptor antagonists and more frequent involvement of the right coronary artery in patients treated by thrombectomy (Table 2).
Characteristics of the procedures performed are shown in Table 3. The Rescue device was the most used (80% of the time). In 7 cases (5%) the device was used, after complications occurred during angioplasty, on a lesion on which thrombectomy was not anticipated. In 5 of these cases the indication for thrombectomy was the occurrence of distal embolization after balloon or stent angioplasty, and in 2 cases due to cessation of flow after stent implantation without the clear presence of thrombus or local complications. In the 5 cases of embolization, the device achieved the complete or partial disappearance of the thrombus on imaging; in only 1 of these cases where flow had ceased was the device able to reestablish flow.
In 83% of cases, the lesion treated was located in the proximal or middle segment of the artery, with the remaining 17% located more distally. On 10 occasions (5 [19%] X-Sizer and 5 [4%] Rescue) the lesion could not be reached with the device. The angulation in the artery origin and the tortuosity of the segment before the lesion were the causes of these failures. In 111 lesions thrombectomy was performed without prior use of another angioplasty device. In 56 cases (50%) a stent was implanted after the thrombectomy without previous balloon dilatation. A final angiographic success rate of 86% was achieved with the procedures. Nosocomial death was principally associated with the presence of shock during the procedure. Ten patients treated in the AMI sinus (8.8%) died prior to hospital discharge (7 of these were in shock before the procedure was initiated). In the 2 patients who died who were not treated for an AMI, the indication for the procedure was cardiogenic shock.
The procedural characteristics and angiographic results are shown in Table 4. Figure 2 shows the changes in the TIMI flow grades during the procedure. In 90 of the 129 cases in which the thrombectomy device was able to reach the lesion (70%), the artery was found to be occluded without anterograde visualization of the distal vessel (Figure 3). Following thrombectomy, the number of patients with TIMI III flow rose from 21% to 70%, and reached 86% at the end of the procedure. Immediately following thrombectomy, 2 patients had worse flow in the treated artery, 9 patients did not improve, and the remaining 116 (91%) had improved flow or the same TIMI III rate of flow that they had previously. Eighty-six percent of the arteries treated with thrombectomy devices had an improved thrombus on imaging, and 67% achieved the disappearance or very substantial improvement of the thrombus.
Fig. 2. Evolution of the TIMI flow grades in the artery treated with the thrombectomy device during the procedure. Only those patients in whom the device was able to access the lesion were included. The post-thrombectomy results do not include 2 patients in whom images were not available after the intervention.
Fig. 3. Percentage of arteries and each of the TIMI flow grades at various phases of the intervention. Only those patients in whom access to the lesion with the device was achieved were included (n=129).
Three (2.1%) patients developed complications attributable to the device. In 1 case, after removing the thrombectomy device (X-Sizer) from a lesion located in the right distal coronary artery, a dissection of the proximal segment was observed and treated with a stent without complications. In the second case, following a successful thrombectomy (Rescue) of the middle third of the descending anterior artery, after removing the device, embolization of a thrombus was observed in the circumflex artery with occlusion of the middle segment. The embolization was treated again with thrombectomy and stent implantation, without other complications. In the third patient, following removal of the device (Rescue), an air embolus was produced in the right coronary artery, followed by transient reelevation of the ST segment and bradycardia, both of which disappeared spontaneously.
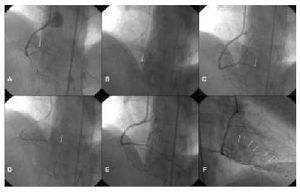
We did not observe a significant delay with the use of thrombectomy as the first device in angioplasty of the AMI sinus. The mean delay from arrival of the patient in the operating room to the achievement of TIMI III flow was 35.4 minutes±19.4 minutes for these procedures, vs 37.4 minutes±82.6 minutes in those cases where thrombectomy was not performed (P=.82). In 2 cases thrombectomy was used only for treatment of the lesion (Figure 4).
Fig. 4. Resolution of thrombotic occlusion with the use of thrombectomy only (Rescue) in a patient with AMI. A: Right baseline angiography. Arrow shows occlusion of the right distal coronary artery (intra-stent). B: Device located immediately proximal to the occlusion. C: Photogram immediately after thrombectomy with resolution of the occlusion of the right distal coronary artery and occlusion of the descending posterior branch. D: Device proximal to the occlusion. E: Final result following the second thrombectomy. F: Final result. Myocardial blush in the area of the right coronary artery.
DISCUSSION
Given the low rate of complications during the procedure (2.1%), the use of the 2 new thrombectomy devices was associated, in our experience, with a clear improvement in angiographic imaging of thrombi and coronary flow, as well as a high percentage of successful final outcomes.
The use of a new device in coronary intervention depends basically on 3 variables: applicability, usefulness, and safety.
Applicability
In the case of our study, both devices were used in a large number of coronary locations and clinical situations with the common denominator of a high suspicion of the presence of thrombus. The incompatibility of the devices in conjunction with 6 Fr catheters (and in the case of the 2-mm X-Sizer device, with 7 Fr catheters) presents a limitation with regard to applicability at a time when the use of 6 Fr catheters has become wide spread. The use of the devices requires advanced planning in most cases, or the replacement of the catheter and arterial introductory catheter once their use has been decided upon during angioplasty. The coaxial character of the X-Sizer device also requires the use of long intracoronary guide catheters or interchangeable devices, requiring, at some points, 2 individuals to manage the procedure.
Although the majority of the lesions treated (83%) were found in the proximal or middle coronary segments (Table 3), access to the lesion by the device was not achieved in 7% of the procedures. The X-Sizer device failed to access the lesion in a greater percentage of cases than the Rescue device (19% of cases vs 4%), which could be related to lesser manipulability of the X-Sizer (the catheter is clearly more rigid) or related to certain types of lesions. The 19% rate is similar to the 17% rate previously noted with the use of the same device.25 On the other hand, use of the device in distal segments was found to be limited by the condition of the coronary arteries in said localization, which makes thrombectomy impossible due to the aspiration opening coming into contact with the actual vessel wall.
With regard to ease of use, both devices are easy to prepare in advance and to use and, once sufficient experience is acquired, the devices can be used in complex clinical situations that require quick, efficient action. In our experience, 111 patients (80%) had an AMI and 14 (10%) were in cardiogenic shock; we did not note a significantly greater delay in restoration of flow in those patients treated with thrombectomy.
Utility
In 86% of cases thrombectomy was effective in decreasing intraluminal thrombi on angiographic imaging; in 67% of cases this reduction was significant and in 28% disappearance of the thrombus was achieved. The technique was proven to be useful in cases in which the thrombus occurred as a complication during the procedure, and a clear improvement or disappearance of the thrombus was achieved in 86% of cases. The final TIMI flow grade was II or III in 99% of patients, III in 86% of patients, without cases of the lack of reflow occurring. We do not know what the contribution of the aspiration devices was to eliminating particles that were not visible on angiography, which might explain the distal microembolization and the phenomenon of the absence of reflow during angioplasty.32,33 Even in the cases that did not improve according to the image of the thrombus on angiography, the usefulness of aspiration devices in these clinical situations cannot be disregarded. It is difficult to assess the real contribution of aspiration on the effect found with both devices. The introduction of a 4.5 Fr to 5.5 Fr catheter may produce a dilatation effect in the artery that may be responsible, at least in part, for the angiographic results observed after their use. On the other hand, the study design, which was observational, did not allow for comparison of the devices with balloon or balloon and stent angioplasty. Up to the present time, although data exists that support the utility of platelet antagonists in patients with unstable coronary syndromes or AMI,11-14,34 even as a rescue strategy in cases of complicated angioplasties,35 the only published studies on thrombectomy devices are preliminary20-25 and, in one case, randomized.19 The data provided by clinical trials currently underway will provide concrete information on the contribution of thrombectomy with these devices to the results of angioplasty in the cases of lesions with a high thrombotic content.
Safety
The devices have been shown to be safe, although they are not exempt from potential complications. In contrast to previous studies,25 we did not observe any incidence of perforation with the devices. The 3 complications noted were resolved during the procedure and did not have adverse consequences. Complications such as those seen in our study and previous studies could possibly be avoided by adequate patient selection, such as selecting patients who do not have tortuous coronary anatomy prior to suffering a lesion, and by slow and careful use of both devices. The opening of the existing valve on the Y key after the removal of the devices encourages evacuation of air from the catheter, avoiding the embolization of bubbles.
Study limitations
The observational nature of the study does not allow comparison of the results of using thrombectomy with both devices to treatment of similar lesions without thrombectomy. It is also not possible to compare the 2 devices, as the choice of one or the other, at the discretion of the interventionist, may be influenced by vessel characteristics and the lesion to be treated as a function of previous experience with both types of device. Both comparisons, which are of great interest, are not the aim of this study, but rather our aim was to describe the applicability and the results of using thrombectomy in daily practice in an interventionist laboratory. Future studies will probably produce the necessary data on both fronts.
Although devices with a distinct mechanism of action are involved (simple aspiration in one case and rotational arterectomy in the other), we presented the data combined since both devices were used to the same end: the extraction of a thrombus. The interpretation of the thrombus image and changes in it, as is true in previous studies, is subjective and, although they were analyzed a posteriori, may be dependent on the specialist involved.
CONCLUSIONS
The new thrombectomy devices, X-Sizer y Rescue, achieve a clear improvement in the angiographic image of the thrombus, and are associated with a high percentage of restoration of normal flow at the conclusion of the procedure with a low incidence of complications. The advantage of these devices over other devices and over conventional treatment with balloon or stent angioplasty need to be analyzed in future studies.
This work was partially financed by the Fundación de Investigación Cardiológica Murciana.
Correspondence: Dr. R. López Palop.
Ricardo Gil, 20, 3.o dcha. 30002 Murcia. España.
E-mail: mlopezs@meditex.es
Received 22 April 2002.
Accepted for publication 3 October 2002.