To determine the current status of anticoagulation control in patients with nonvalvular atrial fibrillation treated with vitamin K antagonists in the primary care setting in Spain.
MethodsThe PAULA study was a multicenter cross-sectional/retrospective observational study conducted throughout Spain. The study included patients with nonvalvular atrial fibrillation who had been receiving vitamin K antagonist therapy during the past year and were attended at primary care centers. International normalized ratio (INR) values over the past 12 months were recorded. The degree of anticoagulation control was defined as the time the patient had remained within the therapeutic range and was determined by both the direct method (poor control < 60%) and by the Rosendaal method (poor control < 65%).
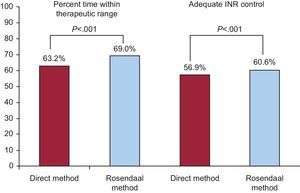
ResultsThe study assessed 1524 patients (mean age, 77.4 ± 8.7 years; 48.6% women; 64.2% in permanent atrial fibrillation; CHADS2 mean, 2.3 ± 1.2; CHA2DS2-VASc, 3.9 ± 1.5, and HAS-BLED, 1.6 ± 0.9). The mean number of INR readings recorded per patient was 14.4 ± 3.8. A total of 56.9% of patients had adequate INR control according to the direct method and 60.6% according to the Rosendaal method. The multivariate analysis identified the following predictors for poor INR control: female sex, dietary habits potentially affecting anticoagulation with vitamin K antagonists, multidrug therapy, and a history of labile INR.
ConclusionsApproximately 40% of patients (43.1% by the direct method and 39.4% by the Rosendaal method) with nonvalvular atrial fibrillation who were receiving anticoagulation therapy with vitamin K antagonists in primary care in Spain had poor anticoagulation control during the previous 12 months.
Keywords
Atrial fibrillation (AF) is the most common arrhythmia in the general population, with an estimated prevalence of about 2%, a figure that rises with age and comorbidities.1 Several studies have recently been conducted in Spain to understand the situation of AF in clinical practice. The VAL-FAAP study2 analyzed nearly 120 000 patients attended at primary care centers and observed that 6.1% of patients had AF. In the general population older than 40 years, the prevalence of AF in Spain was 4.4%.3 Among patients with hypertension and older than age 65 years in the Valencian Community, 10.3% had AF.4
Compared with patients without AF, patients with AF have a 2-fold risk of death and up to 5-fold risk of stroke.5 Atrial fibrillation-related stroke has a higher mortality and a higher risk of recurrences and tends to result in more sequelae.6 In most patients with AF, long-term oral anticoagulation is indicated to prevent thromboembolic complications.2 For this purpose, vitamin K antagonists (VKAs) have been widely used for decades because they can reduce the risk of stroke by about 64%.7
However, VKAs have important limitations that usually condition their use in clinical practice for nonvalvular AF (NVAF).2,8 These include narrow therapeutic window, drug and food interactions, and variable metabolism, which require regular follow-up of anticoagulation status and frequent dose titration.9,10 It is crucial that the international normalized ratio (INR) of patients receiving VKA therapy be within therapeutic range to reduce the risk of thromboembolic and hemorrhagic complications. In fact, time to onset of stroke has been shown to significantly improve in warfarin-treated patients with NVAF and CHADS2 score ≥ 2 compared with untreated patients, but only in treated patients who were within therapeutic range > 70% of the time.11
Therefore, to manage this population adequately, it is essential to identify the degree of INR control among patients with NVAF who are receiving VKA anticoagulation therapy. Several studies12,13 have investigated the degree of INR control in a specific geographic area of Spain or using only a small number of INR values. However, the PAULA study (Perspectiva Actual de la sitUación de la anticoaguLación en la práctica cínica de Atención primaria [Current perspective of anticoagulation in clinical practice in the primary care setting]) was conducted to understand the situation of anticoagulation control over a long period among patients with NVAF who are receiving VKAs in primary care clinical practice throughout Spain. This study specifically looked at anticoagulation in the clinical practice setting at primary care centers.
METHODSThe PAULA study was based on an observational cross-sectional/retrospective, multicenter, national design, and its main aim was to identify anticoagulation control of patients with NVAF who were receiving anticoagulation VKA therapy at primary care centers in Spain last year. The study had the scientific backing of three Spanish primary care societies (SEMERGEN, semFYC, and SEMG).
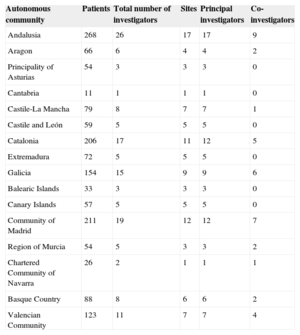
To perform the study, a scientific committee was formed with 2 cardiologists, 1 biostatistician, and 3 general practitioners who were experts in cardiovascular disease and represented the 3 Spanish primary care societies. The general practitioners chose 9 regional coordinators (), who in turn selected 139 investigators () from 99 health centers located throughout the different autonomous communities (except for La Rioja, for logistical reasons) according to the proportion of regional inhabitants, thus ensuring numbers of patients representative of the national territory (Table 1). The regional coordinator initially selected the investigators based on their clinical and research skills, and the choice was then approved by the scientific committee. Each investigator had to include at least the first 10 consecutive patients who met all inclusion criteria and none of the exclusion criteria, who came for routine anticoagulation follow-up, and who gave consent to participate in the study. All patients were recruited between February and June 2014.
Number of Patients Included, Investigators, and Sites According to Autonomous Community
| Autonomous community | Patients | Total number of investigators | Sites | Principal investigators | Co-investigators |
|---|---|---|---|---|---|
| Andalusia | 268 | 26 | 17 | 17 | 9 |
| Aragon | 66 | 6 | 4 | 4 | 2 |
| Principality of Asturias | 54 | 3 | 3 | 3 | 0 |
| Cantabria | 11 | 1 | 1 | 1 | 0 |
| Castile-La Mancha | 79 | 8 | 7 | 7 | 1 |
| Castile and León | 59 | 5 | 5 | 5 | 0 |
| Catalonia | 206 | 17 | 11 | 12 | 5 |
| Extremadura | 72 | 5 | 5 | 5 | 0 |
| Galicia | 154 | 15 | 9 | 9 | 6 |
| Balearic Islands | 33 | 3 | 3 | 3 | 0 |
| Canary Islands | 57 | 5 | 5 | 5 | 0 |
| Community of Madrid | 211 | 19 | 12 | 12 | 7 |
| Region of Murcia | 54 | 5 | 3 | 3 | 2 |
| Chartered Community of Navarra | 26 | 2 | 1 | 1 | 1 |
| Basque Country | 88 | 8 | 6 | 6 | 2 |
| Valencian Community | 123 | 11 | 7 | 7 | 4 |
The inclusion criteria were as follows: a) patients of either sex and 18 years of age or older; b) patients with NVAF who had been receiving VKA therapy for at least the past year at a primary health care center under routine clinical practice conditions; c) patients for whom at least 80% of INR controls were available from the past year, and d) patients who had given written informed consent to participate in the study after they had read and understood the patient information sheet. Patients were excluded if they had cognitive impairment that prevented them from correctly understanding the patient information sheet or informed consent or if they had participated in any clinical trial in the past 12 months.
The study comprised a single visit, that coincided with one of the patient's regular follow-up visits. The data were collected from the medical history and physician interview, but there was no study-specific diagnostic or therapeutic intervention. The data were entered in an electronic case report form. All electronically collected information was checked and reviewed to safeguard the quality of the registry, and the database was cleaned to avoid recording any impossible values. The case report form was designed by a specifically contracted CRO (Contract Research Organization) —Dynamic Solutions—under the supervision of the scientific committee. The CRO was responsible for ensuring the accuracy and quality of the data collected. To handle this, specific telephone follow-up and on-site monitoring were performed as needed. In addition, lists of all data with potential issues were generated and forwarded to the sites and investigators involved.
The following variables were collected: sociodemographic data, relevant medical history, comorbidities, cardiovascular events, including thromboembolic complications and major hemorrhagic events, physical examination, anthropometric data, blood work available from the previous 6 months, AF information (diagnosis date and type of AF), information on AVK therapy (drug and total weekly dose), concomitant treatments, CHADS2, CHA2DS2-VASc, and HAS-BLED scores, INR readings (including date and value), and investigator opinion on patient anticoagulation and possible risk factors associated with worse INR control. Most of the variables analyzed were collected from the patient's medical history. However, some were measured at the visit itself, in particular blood pressure, heart rate, and body mass index. To determine if patients had dietary habits that could affect INR control, patients were specifically asked if they consumed large amounts of foods rich in vitamin K (cereals, broccoli, cabbage, carrots, etc), alcohol, cranberry juice, or ginseng, if they were regular users of phytotherapy, or if they had frequent dietary transgressions. Excessive alcohol intake was considered to be more than 8 alcoholic beverages per week (1 alcoholic beverage was considered to be 10g of pure ethanol).14
To assess patients’ INR control, the time within therapeutic range in the past 12 months was calculated at a centralized site by both the direct method (percent time with INR values within therapeutic range) and by the method described by Rosendaal et al.15 Labile INR was considered to be time within therapeutic range < 60%, according to the definition proposed by Pisters et al.16
The study was approved by the Clinical Research Ethics Committee of the Hospital Universitario la Paz of Madrid. All patients were required to sign the written informed consent before inclusion, once they had read and perfectly understood the patient information sheet.
Statistical AnalysisThe sample size was calculated based on the main endpoint of the study. Previous data on time within therapeutic range showed values with a mean time of 29% to 75%.17,18 In Spain, an earlier study estimated a time within therapeutic range of 64%.19 Conservatively, a value of 60% was assumed for calculation of the sample size. We assumed an alpha risk of .05, a precision of ± 3%, and a loss rate not above 5% to estimate the time within therapeutic range at 60%, with a two-way contrast, which finally yielded a calculation of 1100 patients for inclusion.
For the descriptive analysis, quantitative variables were described with measures of central tendency and scatter (mean ± standard deviation) and qualitative variables were described as absolute (No.) and relative (%) frequencies. In the bivariate analysis to compare 2 means, parametric (Student t test) or nonparametric (Mann–Whitney U test) statistical tests were performed based on the sample distribution; to compare percentages, the chi-square test or Fisher test was used, according to sample size. Bivariate analyses were performed to identify factors individually associated with INR control. Once all factors had been individually identified, a logistic regression analysis was performed. All analyses were performed with SPSS version 18, Data Entry.
RESULTSThe study included 1561 patients, 37 of whom were excluded from the final analysis for different reasons. The causes for exclusion were time from first INR measurement to study start < 1 year (n = 34), history of AF < 1 year (n = 9), improperly recorded data in the electronic case report form (n = 6), and documentation of an insufficient number of INR readings (n = 5). In some cases, more than 1 cause was given for study exclusion. Finally, 1524 patients were included for assessment.
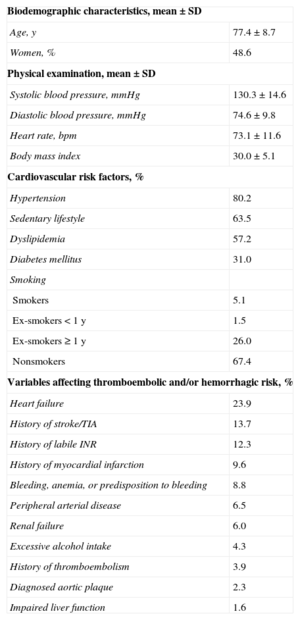
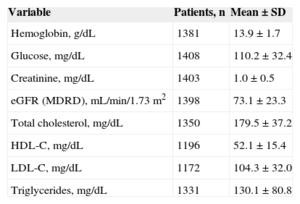
Patients’ baseline characteristics are summarized in Table 2. There was a high number of associated cardiovascular risk factors (80.2% had hypertension, 63.5% was sedentary, and 57.2% had dyslipidemia) and factors that raise the risk of both thromboembolic complications (23.9% had a history of heart failure, 13.7%, stroke/transient ischemic attack, and 9.6%, myocardial infarction) and hemorrhagic complications (12.3% had known labile INR; 8.8%, history of bleeding, anemia, or predisposition to bleeding, and 6.0%, renal failure). A total of 18.4% of patients had dietary habits potentially affecting anticoagulation control with VKAs, 7.7% were taking long-term nonsteroidal antiinflammatories, and 7.5% were receiving other antithrombotic drugs. The total mean number of tablets taken by patients per day was 7.0 ± 3.8. Table 3 lists the most relevant analytical parameters.
Baseline Characteristics of Patients Included in the Study (n = 1524)
| Biodemographic characteristics, mean ± SD | |
| Age, y | 77.4 ± 8.7 |
| Women, % | 48.6 |
| Physical examination, mean ± SD | |
| Systolic blood pressure, mmHg | 130.3 ± 14.6 |
| Diastolic blood pressure, mmHg | 74.6 ± 9.8 |
| Heart rate, bpm | 73.1 ± 11.6 |
| Body mass index | 30.0 ± 5.1 |
| Cardiovascular risk factors, % | |
| Hypertension | 80.2 |
| Sedentary lifestyle | 63.5 |
| Dyslipidemia | 57.2 |
| Diabetes mellitus | 31.0 |
| Smoking | |
| Smokers | 5.1 |
| Ex-smokers < 1 y | 1.5 |
| Ex-smokers ≥ 1 y | 26.0 |
| Nonsmokers | 67.4 |
| Variables affecting thromboembolic and/or hemorrhagic risk, % | |
| Heart failure | 23.9 |
| History of stroke/TIA | 13.7 |
| History of labile INR | 12.3 |
| History of myocardial infarction | 9.6 |
| Bleeding, anemia, or predisposition to bleeding | 8.8 |
| Peripheral arterial disease | 6.5 |
| Renal failure | 6.0 |
| Excessive alcohol intake | 4.3 |
| History of thromboembolism | 3.9 |
| Diagnosed aortic plaque | 2.3 |
| Impaired liver function | 1.6 |
INR, international normalized ratio; SD, standard deviation; TIA, transient ischemic attack.
Analytical Parameters (n = 1524)
| Variable | Patients, n | Mean ± SD |
|---|---|---|
| Hemoglobin, g/dL | 1381 | 13.9 ± 1.7 |
| Glucose, mg/dL | 1408 | 110.2 ± 32.4 |
| Creatinine, mg/dL | 1403 | 1.0 ± 0.5 |
| eGFR (MDRD), mL/min/1.73 m2 | 1398 | 73.1 ± 23.3 |
| Total cholesterol, mg/dL | 1350 | 179.5 ± 37.2 |
| HDL-C, mg/dL | 1196 | 52.1 ± 15.4 |
| LDL-C, mg/dL | 1172 | 104.3 ± 32.0 |
| Triglycerides, mg/dL | 1331 | 130.1 ± 80.8 |
eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MDRD: Modification of Diet in Renal Disease; SD, standard deviation.
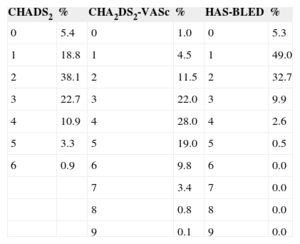
The mean time from AF diagnosis to study date was 6.0 ± 4.2 years. The type of AF was paroxysmal in 20.1%, persistent in 13.6%, and permanent in 64.2%; the type of AF was not documented in the remaining 2.1%. Echocardiograms were available for 81.3% of patients. A total of 75.8% of patients had a CHADS2 score ≥ 2 (mean CHADS2, 2.3 ± 1.2); 94.5% had a CHA2DS2-VASc score ≥ 2 (CHA2DS2-mean VASc, 3.9 ± 1.5), and 13.0% had HAS-BLED ≥ 3 (mean HAS-BLED, 1.6 ± 0.9) (Table 4).
CHADS2, CHA2DS2-VASc, and HAS-BLED Scores for the Study Population (n = 1524)
| CHADS2 | % | CHA2DS2-VASc | % | HAS-BLED | % |
|---|---|---|---|---|---|
| 0 | 5.4 | 0 | 1.0 | 0 | 5.3 |
| 1 | 18.8 | 1 | 4.5 | 1 | 49.0 |
| 2 | 38.1 | 2 | 11.5 | 2 | 32.7 |
| 3 | 22.7 | 3 | 22.0 | 3 | 9.9 |
| 4 | 10.9 | 4 | 28.0 | 4 | 2.6 |
| 5 | 3.3 | 5 | 19.0 | 5 | 0.5 |
| 6 | 0.9 | 6 | 9.8 | 6 | 0.0 |
| 7 | 3.4 | 7 | 0.0 | ||
| 8 | 0.8 | 8 | 0.0 | ||
| 9 | 0.1 | 9 | 0.0 |
CHADS2, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and previous stroke or transient ischemic attack [doubled]; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74 years, and sex category [female]; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly.
Acenocoumarol was prescribed in 94.8% of the patients (n = 1445) and warfarin in 5.2% (n = 79). Anticoagulation follow-up was performed by primary care alone in 70.7%, by hematology in 8.1%, by primary care and hematology jointly in 20.9%, and by cardiology in 0.3%. The total number of INR records in the previous 12 months was 21 982, and the mean number of INR readings recorded per patient in the past year was 14.4 ± 3.8. The percent time within therapeutic range was 63.2% ± 17.9% according to the direct method and 69.0% ± 17.7% according to the Rosendaal method. A total of 56.9% of patients had adequate INR control according to the direct method and 60.6% according to the Rosendaal method (Figure). In 65.0% of patients, the physician considered that the patient had good INR control. Among patients who had poor INR control calculated by the direct method, 30.4% of INR measurements were below 2 and 25.5% were above 3. According to the Rosendaal method, these levels were 30.2% and 25.6%, respectively. In 30.5% of INR readings, the anticoagulant dose was modified. The dose was titrated manually in 46.4% and automatically/mixed (some kind of assistance or help) in the rest.
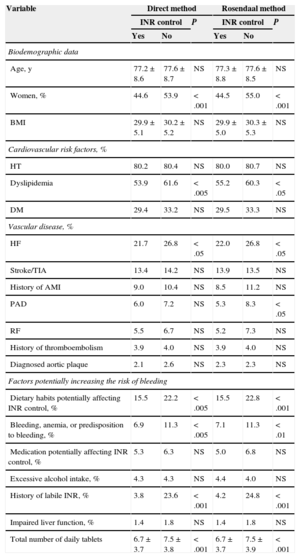
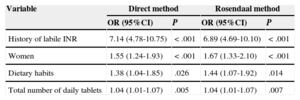
The variables associated with INR control are listed in Table 5 (bivariate analysis) and Table 6 (multivariate analysis) according to the direct method and the Rosendaal method, respectively. In the multivariate analysis, both the direct method and the Rosendaal method found that men had better control than women, that patients with dietary habits potentially affecting VKA anticoagulation were less likely to be well controlled, that patients with poor control took a higher number of tablets per day than those with good control, and that patients history of known labile INR were less likely to be controlled than those who had no known history of labile INR.
Variables Associated With International Normalized Ratio Control (Bivariate Analysis) According to the Direct Method and the Rosendaal Method (n = 1524)
| Variable | Direct method | Rosendaal method | ||||
|---|---|---|---|---|---|---|
| INR control | P | INR control | P | |||
| Yes | No | Yes | No | |||
| Biodemographic data | ||||||
| Age, y | 77.2 ± 8.6 | 77.6 ± 8.7 | NS | 77.3 ± 8.8 | 77.6 ± 8.5 | NS |
| Women, % | 44.6 | 53.9 | < .001 | 44.5 | 55.0 | < .001 |
| BMI | 29.9 ± 5.1 | 30.2 ± 5.2 | NS | 29.9 ± 5.0 | 30.3 ± 5.3 | NS |
| Cardiovascular risk factors, % | ||||||
| HT | 80.2 | 80.4 | NS | 80.0 | 80.7 | NS |
| Dyslipidemia | 53.9 | 61.6 | < .005 | 55.2 | 60.3 | < .05 |
| DM | 29.4 | 33.2 | NS | 29.5 | 33.3 | NS |
| Vascular disease, % | ||||||
| HF | 21.7 | 26.8 | < .05 | 22.0 | 26.8 | < .05 |
| Stroke/TIA | 13.4 | 14.2 | NS | 13.9 | 13.5 | NS |
| History of AMI | 9.0 | 10.4 | NS | 8.5 | 11.2 | NS |
| PAD | 6.0 | 7.2 | NS | 5.3 | 8.3 | < .05 |
| RF | 5.5 | 6.7 | NS | 5.2 | 7.3 | NS |
| History of thromboembolism | 3.9 | 4.0 | NS | 3.9 | 4.0 | NS |
| Diagnosed aortic plaque | 2.1 | 2.6 | NS | 2.3 | 2.3 | NS |
| Factors potentially increasing the risk of bleeding | ||||||
| Dietary habits potentially affecting INR control, % | 15.5 | 22.2 | < .005 | 15.5 | 22.8 | < .001 |
| Bleeding, anemia, or predisposition to bleeding, % | 6.9 | 11.3 | < .005 | 7.1 | 11.3 | < .01 |
| Medication potentially affecting INR control, % | 5.3 | 6.3 | NS | 5.0 | 6.8 | NS |
| Excessive alcohol intake, % | 4.3 | 4.3 | NS | 4.4 | 4.0 | NS |
| History of labile INR, % | 3.8 | 23.6 | < .001 | 4.2 | 24.8 | < .001 |
| Impaired liver function, % | 1.4 | 1.8 | NS | 1.4 | 1.8 | NS |
| Total number of daily tablets | 6.7 ± 3.7 | 7.5 ± 3.8 | < .001 | 6.7 ± 3.7 | 7.5 ± 3.9 | < .001 |
AMI, acute myocardial infarction; BMI, body mass index; DM, diabetes mellitus; HF, heart failure; HT, hypertension; INR: international normalized ratio; NS, not significant; PAD, peripheral artery disease; RF, renal failure; TIA, transient ischemic attack.
Variables Associated with Poor International Normalized Ratio Control (Multivariate Analysis) According to the Direct Method and the Rosendaal Method
| Variable | Direct method | Rosendaal method | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| History of labile INR | 7.14 (4.78-10.75) | < .001 | 6.89 (4.69-10.10) | < .001 |
| Women | 1.55 (1.24-1.93) | < .001 | 1.67 (1.33-2.10) | < .001 |
| Dietary habits | 1.38 (1.04-1.85) | .026 | 1.44 (1.07-1.92) | .014 |
| Total number of daily tablets | 1.04 (1.01-1.07) | .005 | 1.04 (1.01-1.07) | .007 |
95%CI, 95% confidence interval; INR, international normalized ratio; OR, odds ratio.
The results obtained in the PAULA study indicate that INR control in the primary care setting in Spain could clearly be improved. The study included a broad sample that was representative of the entire Spanish population with NVAF attended in primary care, with a high number of INR controls and a long follow-up period. In this study, monitoring of the work was very meticulous and rigorous controls were applied to ensure the quality of the recorded data. Additionally, the study is an example of a joint effort between primary health care physicians and cardiologists and of cooperation between scientific societies, as it is the first time in Spain that a study of these characteristics was backed by the 3 Spanish primary care societies.
The data collected in the PAULA study showed a mean patient age of 77 years; approximately half were women. A total of 80% had hypertension, 24% had heart failure, 14% had a history of stroke or transient ischemic attack, and 10% had a history of infarction. These data indicate that patients with NVAF who are receiving anticoagulation therapy in Spain are relatively older and have a significant number of associated comorbidities. However, compared with other populations with AF analyzed in Spain, particularly those attended in cardiology clinics, the proportion of patients with certain diseases, such as ischemic heart disease or renal failure, was somewhat lower in the PAULA study.2,3,12,13,20 The mean glomerular filtration rate of patients included in the PAULA study was 73.1mL/min/1.73 m2. In populations at higher risk, anticoagulation control is probably even worse.
Regarding the type of AF, several studies have shown that the most common form in patients attended in primary care is permanent AF (45.3% in VAL-FAAP, 77.5% in FIATE, and 64.2% in PAULA).2,13 Therefore, for most patients with NVAF in primary care, it seems that the therapeutic objectives should focus mainly on controlling heart rate and lowering thromboembolic risk.
Risk assessment for stroke and bleeding is fundamental in NVAF. In recent years, the use of scales has become more widespread for both thromboembolic CHA2DS2-VASc) and hemorrhagic risk stratification (HAS-BLED).21 In the VAL-FAAP study, 67.4% had a CHADS2 score ≥ 2 and 85.7% had a CHA2DS2-VASc score ≥ 2.2,8 In the ANFAGAL study12, the CHADS2 score was 2.3; CHA2DS2-VASc, 3.8 and HAS-BLED, 3.1. In the PAULA study, the figures were 2.3, 3.9, and 1.6, respectively. Therefore, anticoagulation indications appear to be on target. The difference in the HAS-BLED score between the ANFAGAL and PAULA studies may be related to the age difference of patients in the 2 studies because the inclusion criteria were somewhat different.
The guidelines21 recommend that patients with AF undergo an echocardiogram as part of the initial study. In the FIATE study,13 67.6% of patients had at least 1 echocardiogram whereas, in the PAULA study, this figure was 81.3%. This finding is highly relevant, as it indicates an improvement in the clinical management of patients with AF in primary care.
Routine INR control was conducted by primary care centers in 72% of patients in the FIATE study.13 In the PAULA study, anticoagulation control was conducted by the primary care physician in 70.7% of patients and jointly by primary care and hematology in 20.9%. Consequently, both studies found that INR control is essentially handled, as is common in Spain, by primary care. However, the prominence of primary care was even stronger in the PAULA study. This is key because although this approach has some important methodological limitations, costs may be lower when INR is controlled at the initial level of health care.22
Inadequate INR control raises the risk of both stroke and bleeding.17,23,24 Therefore, good control is essential. In clinical trials performed with the new direct-acting anticoagulants, the mean time within therapeutic range was 55% to 68.4%.25-28 However, because patients included in clinical trials have restrictive inclusion and exclusion criteria and very rigorous follow-up, the results cannot always be extrapolated to “real life.” In this context, studies conducted in the clinical practice setting provide extremely relevant information.29,30
The ANFAGAL study12 observed a mean of 13.7 readings per patient, 41.5% had less than 60% of controls within therapeutic range, and 42.7% showed a time within therapeutic range < 65%, estimated according to the Rosendaal method. In the FIATE study,13 in patients who were receiving VKA therapy and had only the last 3 INR readings, 66% were within therapeutic range at the last determination and 33% were within range at all 3 readings. Importantly, the ANFAGAL study had a small patient sample and was performed in a single geographic area (Galicia), and the FIATE study had a low number of INR readings. Consequently, the PAULA study data are of considerable interest. In that study, 43.1% of patients had a time within therapeutic range in less than 60% of controls done in the past year and 39.4% had a time within therapeutic range < 65%, with a mean of 14.4 readings per patient. In a meta-analysis of 95 articles, patients receiving anticoagulation VKA therapy remained within therapeutic range 61% of the time, and the control observed in clinical trials or specialized clinics was better than in other settings.31 Therefore, anticoagulation control in Spain is evidently poor, as has been observed in other countries. Unlike other chronic conditions, such as hypertension (where one of the reasons for poor control is a noticeably wrong perception of control), anticoagulation was seen in the PAULA study to be poorly controlled due to the inherent difficulties of maintaining the patient within an adequate therapeutic range, rather than an incorrect perception by the physician.32
Various studies have investigated the factors associated with worse anticoagulation control. The ANFAGAL study observed that poor INR control was more common in patients with high HAS-BLED scores, diabetes mellitus, renal disease, and hypertension.12 In a study performed in the United States, predictors of poor control included alcohol abuse, multidrug therapy, and multiple hospitalizations.33 In another study, the variables associated with good control were regular vitamin K intake, male sex, duration of anticoagulant treatment > 2 months, adequate family support, adequate functional and cognitive ability, and no regular alcohol intake.34 In the PAULA study, the predictors of poor control were history of labile INR, female sex, dietary habits potentially influencing VKA effects, and multidrug therapy. It is important to identify these factors to ascertain which patients may require closer INR control, including those who, at least a priori, may be more likely to potentially benefit from the use of the new direct-acting anticoagulants. For instance, female sex has been associated with worse control in several studies,34,35 probably due to differences in clinical characteristics between the male and female populations.
LimitationsThe limitations of this study are those typical of an observational study. In addition, many variables were collected from the patient's medical history, with the consequent limitations. Nevertheless, the high number of patients included, the meticulous care taken with the quality control of the recorded data, and the rigor of monitoring may have noticeably reduced the effect of these possible limitations. Furthermore, regional coordinators responsible for the study were able to clarify any questions raised and to ensure that all data entry was performed correctly and on time. In addition, the electronic system had an automatic validation program with adequate limit values to ensure the logical coherence of the data and to flag any discrepancies or inconsistencies. The study was based on a retrospective design, but this was an advantage because it provided information on the true clinical setting of anticoagulation control in daily clinical practice whereas in a prospective study, INR controls could be highly influenced by patient inclusion biases. However, because the investigators were recruited as convenient rather than at random, more motivated investigators may have been led to participate and, consequently, the degree of INR control may have been overestimated. Nonetheless, the degree of INR control observed in our study differed little from that reported in other studies, including large clinical trials with new direct-acting anticoagulants. Because the study was designed to determine the degree of INR control in patients treated with VKAs during the past year, some (if not most) patients had been receiving this therapy for years. In fact, the mean time since AF diagnosis was 6 years, although some patients had only been diagnosed in the past year. The degree of INR control differed accordingly, but unfortunately this datum was not recorded because the inclusion criteria merely required access to at least 80% of INR measurements. Although 100% would have been optimal, the reality is that INR control is usually, but not always, done at the same facility due to vacations, trips, etc. In fact, even the large clinical trials do not always have all the data. The inclusion criterion of 80% was a realistic goal to ensure a minimal level of quality. The total number of INR records in the past 12 months was 21 982 and the mean number of INR readings recorded per patient in the past year was 14.4. Likewise, patients were also not recorded if they met the inclusion criteria but decided not to participate in the study. Lastly, the results of this study can only be extrapolated to countries with patients having a similar clinical profile and with a similar health system to ours.
CONCLUSIONSIn clinical practice in Spain, INR control could be greatly improved, as approximately 40% of patients are not well controlled. Predictors of poor INR control were history of labile INR, female sex, dietary habits potentially influencing the effect of VKAs, and multidrug therapy.
CONFLICTS OF INTERESTThis study was sponsored by Bayer Hispania S.L.; however, this sponsor had no influence over the course of the study or the collection or interpretation of the results, even though 2 of the authors are members of Bayer's medical department.
The authors would like to thank the investigators and coordinators for their work, as this study would have been impossible without their assistance.
The authors would also like to thank CRO Dynamic Solutions for their work, which helped ensure the accuracy and quality of the data collected.