Atherosclerosis is a generalized, progressive, chronic condition that can affect the entire vascular tree.1 The clinical manifestations of atherosclerosis are ischemic heart disease, cerebrovascular disease, and peripheral arterial disease. Despite the current prevention strategies and a prompt diagnosis (through diagnostic imaging techniques), atherosclerosis is a major cause of morbidity and mortality in Western societies,2,3 responsible for 84.5% of cardiovascular deaths and 28.2% of deaths due to any cause.4
From a pathophysiological viewpoint, atherosclerosis is a dynamic process that begins with deposition of lipid particles on the arterial wall, followed by apoptosis of the vascular smooth muscle cells and release of matrix vesicles. This process, together with macrophage infiltration, leads to calcification of the arterial wall intima. Fragments of the calcified plaque spread through the surrounding collagen tissue matrix and converge, forming fibrocalcific plaques.5 Thus, arterial wall calcification is considered a direct marker of atherosclerotic disease. Over the last 2 decades, there has been a growing interest in detecting vascular calcification by various diagnostic imaging techniques in asymptomatic individuals with cardiovascular risk factors. The aim is to achieve improved risk stratification and reduce atherosclerotic disease-related morbidity and mortality.
Atherosclerosis is a complex phenomenon, involving numerous factors. An important one is increased blood concentrations of low density lipoprotein cholesterol, which causes changes in capillary permeability and gradual alterations in the arterial walls. Atherosclerosis is more prevalent in older individuals, those with hypertension, smokers, and patients with diabetes mellitus or insulin resistance.6 Atherosclerotic plaques in the aorta have been associated with elevated homocysteine concentrations, prothrombotic markers (prothrombin), proinflammatory markers, (eg, leucocyte count, C-reactive protein concentration), and left ventricular hypertrophy. Although the white population shows a lower prevalence of hypertension and diabetes than the black population, paradoxically, it has a greater atherosclerotic burden.7 There is also some controversy regarding the distribution by sexes: although coronary disease is less prevalent in women than men, there is evidence that women have a higher incidence of aortic atherosclerosis, even at earlier ages and regardless of the presence of cardiovascular risk factors.8
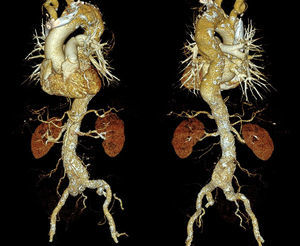
STRUCTURAL AORTIC WALL CHANGES ASSOCIATED WITH ATHEROSCLEROSISAtherosclerotic plaque formation in the aortic wall causes wall erosion and degeneration of the normal media layer. Subsequently, wall atrophy and wall thinning occur, and the vessel dilates in parallel with increases in the volume of plaque.9 Dilation is considered a compensatory mechanism to prevent or delay stenosis of the vessel lumen, but in arteries having a medium-sized or small diameter and a large atherosclerotic burden, it is sometimes insufficient and stenosis ensues. In larger vessels, however, dilation maintains the vessel lumen open for a longer time, avoiding stenosis but producing a risk of aneurysm formation (Figure 1).
Changes in the aortic media layer are also associated with a decrease in the elastic properties and distensibility of the vessel. In this regard, non-oscillatory shear stress on the vessel wall has been linked with greater fat infiltration and cholesterol-rich plaques, which perpetuate the dilation process. Aortic calcifications originate in vessel segments where the wall stress is less intense, but highly ocillatory. The lesser curvature of the aortic arch and the posterior wall of the descending aorta are the most commonly affected areas.
Another factor associated with vascular dilation is the vessel wall tension. Wall tension is proportional to the radius of the vessel; thus, with gradual thinning of the arterial wall, the tension produced suffices to cause a slow increase in the vessel diameter. This increase then leads to an increase in wall tension, resulting in a vicious circle of vascular atrophy and dilation. This mechanism explains why the descending aorta and abdominal aorta are more prone to develop aneurysms than the coronary or carotid arteries.
The medial layer of the vessel segments most proximal to the aorta contains vasa vasorum. These structures, which vascularize the vessel wall layers, are not found in the more distal segments. Hence, when atherosclerotic plaques are present, impeding proper diffusion and irrigation of the vessel wall from the lumen, hypoperfusion can occur in this region. This reduction in wall perfusion also facilitates atrophy and progressive dilatation, and helps to explain the greater frequency of aneurysms in the abdominal aorta.10
The aortic flow pattern is another factor that has an influence on aortic dilatation. A clockwise helicoidal flow pattern has been described at the level of the aortic arch that protects against plaque formation in the distal arch and the proximal descending aorta. In addition, the aorta shows a gradual physiologic narrowing known as aortic taper, in which the ascending aorta has a larger diameter than the descending aorta. This results in an acceleration of blood flow in the descending aorta, which avoids sluggish flow and the development of atherosclerosis. However, when the descending aorta progressively dilates as a consequence of diffuse aortic atherosclerosis, the blood flow slows and this favors progression of the atherosclerotic plaques.11 Atherosclerosis, together with changes in the aortic wall composition and elasticity, lead to elongation and tortuosity of the aorta.12 These alterations in the morphology and configuration of the vessel are associated with wall tension and flow distribution changes, which enhance the degenerative process.
Thus, there is a relationship between atherosclerosis and changes in aortic morphology. In a recent study in Revista Española de Cardiología, Craiem et al. were the first to describe a correlation between aortic calcification and the diameter of the aortic arch and descending aorta. These authors also found that the ascending aorta diameter did not correlate with the total aortic calcium, suggesting that aortic dilation may have different pathophysiologic mechanisms and require different prevention strategies.13
Despite considerable therapeutic advances in secondary prevention of atherosclerotic disease, achieving a prompt diagnosis of the condition in asymptomatic patients (ie, primary prevention) remains a challenge. That is why huge efforts have been made in recent years to develop and apply noninvasive diagnostic imaging techniques to promptly detect atherosclerotic disease.
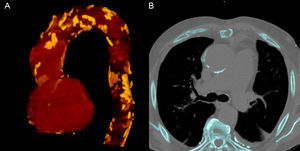
DIAGNOSTIC IMAGING TECHNIQUES FOR EARLY DETECTION OF ATHEROSCLEROSISBased on the relationship between vascular calcification and atherosclerotic disease, several diagnostic imaging techniques have focused on detection and measurement of vascular calcification as a marker of the degree and severity of atherosclerosis. Radiologic study of vascular calcification by fluoroscopy was described at the end of the 1950s, and soon after, an association was established between vascular calcification and episodes of cardiovascular disease.14 The later development (in the 1980s) of electron beam computed tomography and mutidetector computed tomography enabled vascular calcification to be precisely diagnosed and measured (Figure 2A).
Vascular calcium is measured using the Agatson score, which is the product of the area of each calcification (in pixels, mm2) having computed tomographic attenuation of at least 130 Hounsfield units and the density score, obtained by rating the lesion on a scale.15 The Agatston score has been widely used for studying coronary atherosclerosis16 because it provides important prognostic information, as will be seen later on.
In an effort to establish prognostic factors and markers of cerebrovascular and peripheral vascular disease (in addition to ischemic heart disease), use of the Agatston score has been extended to other levels of the vascular tree (Figure 2B). However, the imaging protocol for calculating the score (eg, slice thickness, kilovoltage) has only been standardized for measuring coronary calcification. Standardized protocols are lacking for assessment of aortic calcium, and this can lead to considerable variation.
In determining the coronary calcium score, the software used in the analysis can generate a certain degree of variability in the results. Nonetheless, comparisons of intra-scanner measurements obtained with the same system as well as inter-scanner measurements have shown quite high agreement.17 The variability is greater, however, when scoring aortic calcium: the score value increases as slice thickness decreases, and other changes occur when the remaining imaging parameters used in the reconstruction are modified. Craiem et al. established a standardized protocol for quantifying aortic calcification by computed tomography using 2.5-mm slice thickness. The authors determined the calcification burden in total and by sections, after dividing the aorta into 5 anatomic segments: segment 1, from the sinotubular junction to the pulmonary bifurcation; 2, from the pulmonary bifurcation to the brachiocephalic trunk; 3, from the brachiocephalic trunk to the left subclavian artery; 4, from the left subclavian artery to the descending aorta at the level of the pulmonary bifurcation; and 5, the thoracic descending aorta from the pulmonary bifurcation to the coronary sinus.18
This observational study found that 60% of aortic calcification is concentrated in the aortic arch and the proximal descending aorta. The presence of aortic calcium was considered a prognostic risk factor beyond the conventional factors, able to define a subgroup of patients who would benefit the most from intensive treatment and close follow-up.18
VASCULAR CALCIFICATION AND PROGNOSTIC IMPLICATIONSThe importance of scoring vascular calcium resides in the prognostic implications of the scores obtained in the follow-up of patients with classic cardiovascular risk factors. Based on the calcium score, patients with intermediate cardiovascular risk can be reclassified to the high-risk group. In early studies, coronary calcium detected by fluoroscopy in patients with coronary disease was associated with shorter survival during follow-up14 regardless of the severity of the stenosis seen on coronary angiography. Since that time, numerous studies have demonstrated the prognostic value of the calcium score in asymptomatic patients with an intermediate risk of coronary disease.
In a progressive manner, as in the Framingham risk scale, the risk for a non-fatal infarction or cardiovascular death is 3.9 times greater in individuals with an Agatston score of more than 300 than in those with a score of 0. These findings have been corroborated by other observational studies showing that an elevated calcium score is linked with higher cardiovascular mortality, even after adjusting for the classic cardiovascular risk variables,19,20 as a high score is often associated with the presence of significant coronary disease. Furthermore, since atherosclerosis risk increases with age, an Agatston score higher than 615 in persons older than 65 years would classify them as being at high risk, whereas a score below 50 would reclassify them into the low-risk population.
Atherosclerosis is considered a systemic disease, and various studies have reported an association between coronary calcification and vascular disease at other levels. For example, coronary artery disease is more severe in patients showing greater aortic calcification.21 This association has led to an increasing interest in detecting vascular calcifications beyond those occurring in the coronary arteries. The FAPS study reported that patients with atherosclerotic plaques in the distal ascending aorta and aortic arch had a higher stroke rate than those with atherosclerosis of the descending thoracic aorta.22 Later on, a report from the MESA study, which assessed the correlation between coronary calcification and carotid intima-media thickness, found that the coronary calcification score showed a better correlation with the total of cardiovascular episodes, whereas intima-media thickness correlated better with cerebrovascular episodes.23
A recent study by Bos et al. in 2408 asymptomatic patients older than 55 years reported that extension of vascular calcification beyond the coronary vessels was associated with a higher overall mortality rate regardless of the classic cardiovascular risk factors. In addition, calcium in the aortic arch was linked with a higher rate of cardiovascular mortality.24
There is considerable interest in the prognostic value of calcification at different levels of the vascular tree, as the additional data obtained by diagnostic imaging will provide more comprehensive information on cardiovascular risk. The ultimate aim is to achieve earlier and better risk stratification in the asymptomatic phase, which will help to guide the management of these patients.
However, despite this growing interest, there are several scientific gaps that deserve further study. First, the optimal imaging protocol is uncertain and the extent of the vascular tree that should be examined is unknown. The advantages of prognostic stratification must be weighed against the inherent risk of radiation exposure, which is low but not negligible, considering the magnitude of the entire vascular tree. Nor is it known how often these examinations should be carried out to provide a close follow-up and proper monitoring of the response to treatment. Lastly, the age range of the population that might benefit the most from these imaging studies also needs to be defined. In older patients, age itself and determination of the cardiovascular risk factors would likely provide sufficient prognostic data, while the diagnostic imaging findings would not offer additional information.
In conclusion, aortic atherosclerosis leads to changes in the morphology and configuration of the aorta. In turn, these changes are an additional factor favoring progression of the atherosclerotic disease. Therefore, early detection of atherosclerosis in the subclinical phase would enable initiation of measures to slow its progression and improve the prognosis of patients with cardiovascular risk factors. Although further scientific evidence is needed, the current data indicate that more complete detection of vascular atherosclerosis beyond the coronary arteries will provide prognostic information of interest that can be used to guide the clinical management of these patients.
CONFLICTS DE INTERESTNone declared.