Bicuspid aortic valve (BAV) is the most common congenital cardiac abnormality, occurring in 0.5%-1.4% of the population; this anomaly is sporadically transmitted genetically by an autosomal-dominant pathway, with a 3:1 male predominance.1 BAV is clinically important, not only because of valve-related complications (valve dysfunction, infective endocarditis), but also because of its association with many vascular abnormalities, including aortic dilatation2 (Figure 1).
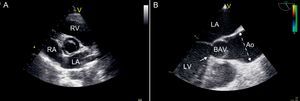
Figure 1. Echocardiographic images assessing bicuspid aortic valve. A: Transthoracic parasternal short-axis view showing bicuspid aortic valve with antero-posterior configuration. B: Transesophageal long axis view showing abnormal systolic opening (“doming”) of a bicuspid aortic valve. Aortic dilatation is evident, starting above the sinotubular junction and being maximal in the mid-ascending portion of the tubular aorta (dotted line). Subaortic stenosis is present, with a fibrous ridge arising from the septal portion of the left ventricular outflow tract (arrow). Ao, aorta; BAV, bicuspid aortic valve; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The reported prevalence of BAV-related aortic dilatation ranges from 33%-80%. This variation is based on thresholds used to define dilatation, normal values for age and body surface area, and region of the aorta. Different types of aortic aneurysms have been described depending on the region involved,3 being the dilatation of the mid ascending tract (dilatation at the tubular ascending portion alone) the most frequent pattern observed.4 Age and BAV morphology have also been associated with ascending aorta (AscAo) dimension, although the role of morphology is not clearly defined.5, 6
PathophysiologyThere is continuing controversy between 2 theories on the pathogenesis of aortic dilatation in patients with BAV. One theory argues that AscAo dilatation may be a consequence of blood flow turbulence, with a primary hemodynamic effect acting from fetal life and resulting in different degrees of stress-induced aortic degeneration. Although this hypothesis has the advantage of relative simplicity, some studies suggest that hemodynamic alterations alone cannot be solely responsible for aortic dilatation in these patients.7 The second theory raises the hypothesis of the presence of an inborn congenital defect in aortic structure. The association between cusp arrangement and AscAo disease may be explained by abnormal development patterns of neural crest cells, and structural abnormalities would occur at cell level regardless of the hemodynamic lesion.8 This theory has become increasingly popular over the last decade, and has led to more aggressive recommendations for the treatment of the proximal aorta in these patients. There are some lines of evidence supporting the predominant congenital wall abnormality:
• In BAV patients (including children) with normally-functioning aortic valve, significant proximal aorta enlargement has been observed compared with age-matched normal controls. However, a ‘normally’-functioning BAV is intrinsically stenotic, with nonaxial and turbulent flow even if there is no transvalvular pressure gradient. This flow is highly eccentric, causing abnormal helical flow patterns in the proximal aorta.9 These abnormal hemodynamic patterns act over long periods of time and may lead to asymmetric stress-induced aortic wall lesions with subsequent dilatation of specific aortic segments.
• Patients with BAV have been shown to have larger aortic root and AscAo diameters than patients with tricuspid aortic valve (TAV), even after matching for hemodynamic severity of valvular lesions.5 However, jet eccentricity that occurs through BAV has not been analyzed.
• AscAo dilatation can occur even after aortic valve replacement.10
• An intrinsic wall abnormality would be supported by the demonstration of a histopathological abnormality underlying AscAo complications in BAV, namely cystic medial degeneration. This pathologic finding has been observed in the aortas of patients with BAV before aneurysm formation occurs, and consists of a reduced extracellular matrix component and increased matrix degradation enzymes in the aorta.11, 12, 13 Interestingly, although similar histological and biomolecular changes occur in BAV and Marfan aortas, the distribution of these changes differs in both conditions, and even in TAV aneurysms (Table 1), which advocates a primary role of hemodynamics in the development of reactive aortic wall remodeling.
Table 1. Histopathology of Cystic Medial Degeneration
Characteristic BAV aneurysm Marfan aneurysm TAV aneurysm Reduction ECM components Increase VSMC apoptosis: reduction production of ECM proteins Elastin fragmentation: loss of structural support and elasticity FB-1 deficiency: detachment of VSMC from elastin and collagen matrix (matrix disruption) Lesser degree of changes Increase matrix degradation enzymes Increase MMP-2Increase MMP-9Increase MMP2/TIMP-1 Increase MMP-12 Increase MMP-13 Spatial CMD distribution Asymmetrical Circumferential Confined to aneurysmal region BAV, bicuspid aortic valve; CMD, cystic medial degeneration; ECM, extracellular matrix; FB, fibrillin; MMP, matrix metalloproteinase; TAV, tricuspid aortic valve; TIMP, tissue inhibitor of metalloproteinase; VSMC, vascular smooth muscle cells.
• Finally, it has been suggested that BAV disease has high heritability, with determination being almost entirely genetic. In large family studies, the prevalence of BAV in first-degree relatives (FDR) of an individual with BAV has been reported to be 9%. In addition, some studies have reported aortic root dilatation, thoracic aortic aneurysm or aortic dissection in up to one third of FDR of BAV patients whether or not a BAV was present.14, 15 Although multiple potential gene sites for BAV and AscAo aneurysms have been suggested, no definite site has been firmly established as being responsible for aortic dilatation in BAV.
Aortic dilatation has been documented in childhood, which suggests that this process begins early in life. Information on the rate of progression of aortic disease associated with BAV varies widely, with studies reporting ≈0.3–1.1mm per year (mm/year).7 In the Olmsted County study, the prevalence of AscAo dilatation (>40mm) was 15%, and in a subset of patients with repeat measurements, the prevalence rose to 39%.16 Although numerous risk factors are associated with dilatation of the aorta (high blood pressure, male sex, significant valve disease), the most important variable is probably age. Both pediatric and adult studies have reported that, compared with TAV-associated aneurysms, the BAV-related aneurysm becomes enlarged more rapidly and presents at a significantly younger age.6, 7 In addition, progressive dilatation of the aorta is more common in patients with larger aortas at baseline.
Aortic Dissection and RuptureAlthough the most feared aortic complication in BAV patients is aortic dissection, the actual incidence of this complication remains under debate. The prevalence varies depending on the cohort studied, with a pooled estimate of cases of 4%.17, 18 Some reports from referral centers suggested an aortic dissection risk 5-9-fold higher in BAV than in TAV, whereas others observed no such association.7, 19 Recent studies have yielded a lower risk. In the Toronto series,20 the prevalence of dissection was 0.1% per patient-year of follow-up, and in the Olmsted County study, the 25-year cohort risk of aortic dissection after echocardiographic diagnosis was 0.5%.21
In fact, BAV-associated AscAo aneurysms dissect and rupture with a size range comparable to that of aneurysms of other etiologies (mean: 60±12mm; range: 30-108mm).22 The increased risk of dissection and rupture associated with BAV is due to the higher prevalence and rate of aortic dilatation, which occurs at a significantly younger age than does idiopathic AscAo aneurysms.7, 19 A comparison between BAV and TAV patients showed that, although BAV patients had a higher rate of aortic growth (1.9 vs 1.3mm/year), the incidence of rupture and dissection was similar.7 Therefore, despite faster growth rates, negative events occur at similar rates and at similar aortic diameters.
Patients with Marfan syndrome have a much higher lifetime likelihood of aortic dissection (40%) than patients with BAV. However, since BAV disease is ≈100 times more common than Marfan syndrome, BAV disease is responsible for an equal or greater number of aortic dissections than Marfan syndrome.22
Diagnosis and SurveillanceTransthoracic echocardiogram (TTE) is usually the primary imaging technique for diagnosing BAV (Figure 1), since it identifies patients in whom the aortic root or AscAo is enlarged and assesses their progression over time. The normal range (AscAo and aortic root diameter<21mm/m2) has to be corrected for age and sex. These TTE measurements correlate closely with measurements by multidetector computed tomography (CT) scan23 and magnetic resonance imaging (MRI),24 suggesting that TTE is an accurate imaging modality. Nevertheless, standard TTE may not visualize the entire AscAo and may fail to detect its largest diameter, typically most pronounced in the proximal to mid-AscAo. It is recommended that an MRI or CT scan be performed to evaluate the entire AscAo when it is not adequately visualized by echocardiogram. It is also reasonable to perform a baseline MRI or CT scan when aortic dilatation (≥45mm) is first diagnosed, which would serve as a reference measure during the follow-up if discrepancies among serial echocardiograms are encountered. An MRI scan can accurately detect and measure aortic aneurysms and confirm valve anatomy, avoiding contrast and radiation exposure. Recently, abnormal systolic helical flow has been demonstrated by 4-dimensional MRI, and the degree and direction of flow jet eccentricity may be crucial for determining the risk of segmental aneurysm formation.9 The specific aortic anatomy may dictate which imaging study is optimal. For example, when aneurysms involve the aortic root, MRI is preferable to CT, because CT images the root less well and is less accurate in sizing its diameter. If there is a contraindication to CT and MRI, TEE is a reasonable alternative that is clearly superior to TTE for assessing aneurysms located in the aortic root (Figure 1), aortic arch and descending aorta. The combination of TTE and multi-slice CT angiogram may provide all the information required if surgery is planned.
When a thoracic aortic aneurysm is first detected, it is not possible to determine its rate of growth, and it is therefore appropriate to obtain a repeated imaging study 6 months after the initial study. If the size of the aneurysm remains unchanged, it is then reasonable to obtain an imaging study on an annual basis in most cases.25 This also applies after aortic valve replacement, as progressive aortic dilatation can occur. Follow-up evaluation should be considered at shorter intervals depending on aortic dimensions, rate of expansion and physical activity. In BAV patients without significant valve lesions and normal aortic diameter, an echocardiogram every 2 years may be sufficient.
Finally, screening of FDR of BAV patients should be considered to detect aortic valve malformation and dilated AscAo. TTE may reliably identify FDR with structural cardiac abnormalities14, 26 (Figure 2). However, the natural history of FDR with a mildly dilated aortic root and a TAV phenotype is unknown. Therefore, long-term follow-up studies of this population are needed, both to determine the rate of dilatation, and in turn, to establish the frequency of serial TTE screening, which would reasonably detect aortic dilatation before complications occur.14
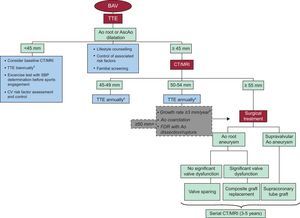
Figure 2. Algorithm showing the general approach to aortic dilatation management and surveillance. Ao, aorta; AscAo, ascending aorta; BAV, bicuspid aortic valve; CT, computed tomography; CV, cardiovascular; FDR, first-degree relatives; MRI, magnetic resonance imaging; SBP, systolic blood pressure; TTE, transthoracic echocardiogram.aConsider earlier follow-up if valvular dysfunction is present.bSerial comparisons of images made with the same imaging technique, side by side, at the same aortic level.
Management Medical ManagementIn addition to routine imaging assessment, patients with BAV should receive information on the risk of dissection and aneurysm formation, as well as the typical symptoms of acute aortic dissection (Figure 2).
Activities or lifestyle should be modified since high intensity, competitive and collision sports are potentially dangerous and may precipitate aortic dissection or rupture in more than mild dilated aortas (45mm).27
Associated cardiovascular risk factors such as high blood pressure should be aggressively controlled in these patients. β-Blockers may be administered to delay or prevent aortic root dilatation or progression in BAV patients (in the absence of severe aortic regurgitation).25, 26 However, the rationale for this recommendation is based on the effect of β-blocker treatment in Marfan,28 and should therefore be confirmed by further research. Although some studies yielded a benefit of angiotensin receptor blocker and angiotensin-converting enzyme inhibitor treatment in people with Marfan syndrome,29, 30 there are currently no data demonstrating a similar benefit from these therapies in BAV aneurysm disease.
The medical management of BAV disease does not currently include statins, which may potentially limit aortic dilatation by reducing matrix metalloproteinase expression and improving endothelial function via increased endothelial nitric oxide synthase.31 A recent randomized, placebo-controlled trial involving participants with mild or moderate aortic stenosis showed that statins did not alter aortic valve-related events or progression of aortic valve stenosis.31 However, only ≈5% of the study participants had BAV. Further research focused on the effects of statins on AscAo dilatation, dissection and rupture in patients with BAV disease is warranted.
SurgeryThe optimal timing of aortic surgery in patients with BAV without indication for valve surgery remains uncertain owing to the limited data available on the natural history of asymptomatic aortic dilatation (Figure 2). AscAo diameter is the dimension most often used to determine the size of the enlarged aorta and is a major criterion for recommending elective surgery in asymptomatic patients with aortic aneurysm. Current guidelines state that surgery to repair the aortic root or replace the AscAo is indicated in patients with TAV if the diameter of the aortic root or AscAo is≥55mm, and that formulas that incorporate height and aortic cross-sectional area for patients of small stature should be used.25, 32, 33 Lower threshold (≥50mm) is recommended in Marfan and BAV patients. However, based on recent long-term follow-up studies, the differences between both entities are significant. Patients with BAV probably fall between the 2 extremes of a spectrum of conditions represented by Marfan syndrome and degenerative AscAo aneurysm, and size criteria for surgical intervention may be midway between those established for both conditions (50 and 55mm, respectively). In BAV patients with risk factors such as aortic coarctation, severe aortic stenosis or FDR with a history of aortic rupture or dissection, surgery is advisable when AscAo is≥50mm, and in those undergoing elective aortic valve replacement when is ≥45mm. The rate of expansion has been shown to be another major predictor of rupture. A growth rate over 10mm/year has been traditionally considered as indication for surgery.34 Recent advances in the field of cardiovascular imaging have improved the reproducibility of serial measurements. Consequently, there is general agreement to accept an expansion rate more than 3mm/year as the cut-off value to indicate surgery, provided that comparisons have been accurately made side by side, with the same imaging technique, and at the same level of the aorta. Finally, intervention criteria must be carefully weighed against surgical risk, both for the patient (age, comorbidities, etc.) and for the center. At the best centers, current hospital mortality for elective surgery of the AscAo in young patients without comorbidities is around 2%.34, 35 Patient characteristics, aortic valvular dysfunction, location of aortic dilatation and type of surgery should be considered in the decision making process.
There are several possible surgical options, and the choice depends on the location of the aneurysm, the distal extent of aortic involvement and the desired anticoagulation status (Table 2).36, 37 If valve repair or a valve-sparing intervention is considered, TEE may be performed pre- or intraoperatively to define the anatomy of the cusps and AscAo.38
Table 2. Surgical Options for Bicuspid Aortic Valve Disease
| Surgical technique | Considerations |
| Reduction aortoplasty with /without external synthetic wrapping | • Generally not recommended (risk of recurrent dilatation)• Alternative for patients with high surgical risk (especially if sinuses are not significantly dilated) |
| Replacement of aortic root and AscAo with reimplantation of coronary ostia (Bentall procedure) | Standard technique in patients with significant valve disease and dilatation of AscAo |
| Aortic valve replacement and separate supracoronary aortic repair | • Generally not recommended (risk of progressive sinus dilatation)• Alternative for older patients with BAV stenosis, normal sized sinuses and dilatation of supracoronary AscAo |
| Valve-sparing aortic replacement | Acceptable option for young patients with normally-functioning BAV |
| Pulmonary autograft | • Generally not recommended (risk of autograft dilatation)• Alternative for children, adolescents or young women who wish to become pregnant |
| Aortic valve repair | Acceptable option if careful patient selection is made |
AscAo, ascending aorta; BAV, bicuspid aortic valve.
BAV disease has a high prevalence of AscAo dilatation, and warrants aggressive control of hypertension and follow-up by imaging techniques. Once the AscAo diameter reaches 45mm, annual imaging by echocardiography is indicated. Alternatively, MRI or CT can be considered, especially as a baseline study that would serve for further comparisons whenever needed during follow-up. Although there are limited data on prophylactic intervention, it is suggested that elective surgical repair of BAV-associated aortic dilatation should be recommended more aggressively, at a diameter of 50-55mm depending on patient characteristics and the presence of risk factors, and at 45mm in those with concomitant indication for aortic valve replacement.
Furthermore, screening by echocardiography should be offered to FDR of patients with BAV, as the chance of finding significant silent valvular or aortic malformations is substantial, and early diagnosis might prevent the morbidity and mortality related with them.
Conflicts Of InterestNone declared.
Corresponding author: Servei de Cardiologia, Hospital Vall d’Hebron, P.° Vall d’Hebron 119, 08035 Barcelona, Spain. aevangel@vhebron.net