There is currently no other hot topic like the ability of current technology to develop capabilities similar to those of human beings, even in medicine. This ability to simulate the processes of human intelligence with computer systems is known as artificial intelligence (AI). This article aims to clarify the various terms that still sound foreign to us, such as AI, machine learning (ML), deep learning (DL), and big data. It also provides an in-depth description of the concept of AI and its types; the learning techniques and technology used by ML; cardiac imaging analysis with DL; and the contribution of this technological revolution to classical statistics, as well as its current limitations, legal aspects, and initial applications in cardiology. To do this, we conducted a detailed PubMed search on the evolution of original contributions on AI to the various areas of application in cardiology in the last 5 years and identified 673 research articles. We provide 19 detailed examples from distinct areas of cardiology that, by using AI, have shown diagnostic and therapeutic improvements, and which will aid understanding of ML and DL methodology.
Keywords
Few topics are as consequential, even in medicine, as the potential ability of modern technology to develop capabilities similar to those of human beings. The ability of machines or computer systems to mimic human intelligence processes is called artificial intelligence (AI). AI is becoming highly advanced in other professional disciplines and our current challenge is to transfer all of this development to the medical field and specifically to cardiology.
This article aims to clarify for the reader various terms that may still seem foreign, not just AI, but also machine learning (ML), deep learning (DL), data science, and big data, and to describe in detail the concept of AI and its types, the learning techniques and methodology used by ML, cardiac imaging analysis with DL, the contribution of this technological revolution to classic statistics, and its current limitations, legal aspects, and, critically, initial applications in cardiology.
DATA SCIENCEThe terms data science, big data, AI, and ML have different meanings, despite belonging to the same discipline: the analysis and extraction of information from data. However, these concepts are sometimes used interchangeably and in ambiguous ways.
The term big data was first coined in 2005 by R. Magoulas, who described it as a massive volume of data that, due to size, overwhelms traditional storage and processing software. In turn, the world of big data revolves around 7 Vs: data volume, velocity, variety, veracity, validity, volatility, and value. That is, big data generate information at high speed, controlling the integrity of the data and exhibiting huge variety in terms of nature and type (eg, text, images, videos, different systems, and different providers). In cardiology, big data would be viable within a framework of national, European, or global collaboration, involving data homogenization and sharing by hospital entities to create large-volume repositories. These large databanks can support our daily practice by helping to establish protocols, promote standardized and early disease diagnosis, aid prognosis of disease progression, and support treatment planning for our patients.1
This type of information processing would not be possible without recent technological advances in AI. AI uses tools able to identify existing patterns in data. It was defined for the first time in the 1950s and encompasses multiple subdisciplines, from expert systems and robotics to ML. It is precisely this last term that we will focus on in this review due to the major advances in the field in recent years.
The term machine learning was first coined in 1959 by AL Samuel.2 Its objective is to develop algorithms enabling computer systems to make decisions and learn from the results; these systems would thus be able to learn to do something without having been explicitly provided with the programming required.
Among the many ML techniques, there is more and more interest in DL models.3 These base their predictive power on artificial neural networks (NNs) and are characterized by having multiple layers of information processing (transformations) that allow them to analyze datasets with more complex patterns. These algorithms are enabling pivotal advances in speech and image recognition; the latter is particularly important in cardiology.
The data science field encompasses all aspects related to the derivation of the existing information contained in data. It involves scientific methods, processes, and systems to extract knowledge or develop a better understanding of data, normally applying novel processing techniques such as AI. Although AI and ML are often used as synonyms, they are different. AI covers a broader scope of this technological revolution and includes both ML and DL (figure 1A). The right-hand panel of figure 2 illustrates the interest triggered by these concepts in the last 3 years, with increasing interest in ML, AI, data science, and DL. Big data is the most common term In Spain, southern Europe, and South America, maching learning in the United States, Canada, the United Kingdom, northern Europe, and Australia and deep learning in China and Japan.
A: Venn diagram of the most commonly used terms in the data science disciplines. B: total searches (source: Google Trends) in the last 5 full years of terms related to artificial intelligence and data science; the vertical axis of the diagram represents the proportion of a topic with respect to the total number of searches on the topics. C: the most searched term in each country in the same period.
Nowadays, it is difficult to find a universal definition of what is known as AI. The term itself is often applied to the field of computer science that tries to mimic human cognitive processes, learning capacity, and knowledge storage. Other definitions are broader and encompass the understanding and construction of intelligent entities, generally understood as computer software. In terms of types of task, AI can refer to automated systems capable of, for example, translating a document, recognizing people by their facial features, or driving a car. However, AI is not limited to imitating human tasks: in some cases, it is able to beat the best expert in a field by making decisions with lower error rates than humanly possible or by identifying patterns imperceptible to the human eye.4 Therefore, AI allows information to be analyzed with a distinct approach from the traditional one. Thus, when responding to an event, we are no longer limited to describing the available information. AI allows other questions to be answered: what happened? (diagnosis), what will happen? (prediction), and what should I do? (prescription).
The most important application areas of AI in the health care field include the following: automatic speech recognition and natural language processing; prediction, recommendation, and diagnostic algorithms; computer vision and image analysis; robotics; and AI and expert systems.
Automatic speech recognition and natural language processingThe aim of these disciplines is to develop mechanisms for communication between people and machines using natural language. In the medical field, automatic speech recognition is already being used to record patients’ clinical information.5 In addition, natural language processing is allowing, as already seen in several examples in Spain in the field of cardiology, disease classification and the selection of the most appropriate cohort for a clinical study by analyzing medical record registries.6
Prediction, recommendation, and diagnostic algorithmsThis is probably the most mature area in the worlds of medicine and cardiology and of ML and DL. Interest primarily lies both in the automation of repetitive tasks, such as the evaluation of diagnostic tests, and in knowledge generation through clinical data analysis. A major part of this article focuses on describing the methodology to be followed in this type of study and introducing use cases in cardiology.
Computer vision and image analysisThis scientific discipline encompasses methods to acquire, process, analyze, and understand real-world images in order to produce numerical or symbolic information that can be processed by a computer. These techniques have undergone a major revolution in recent years due to the application of DL algorithms and it is one of the disciplines making the greatest contribution to medicine today, and therefore to cardiology, as detailed below.
Robotics and artificial intelligenceIn robotics and AI, the objective is to build physical systems with intelligent behavior. This field has undergone many years of development but is experiencing a boom in the various areas it includes (eg, automatic speech recognition, computer vision). In the cardiovascular area, its development has been ongoing for years in the field of surgery, with a clear example being the Da Vinci surgical system.7
Expert systemsIn AI, an expert system is software that emulates the decision-making ability of a human expert. These systems are based on rules or even clinical cases. For some time, expert medical systems have been available that try to simulate the reasoning of specialists and provide the probable diagnosis and optimal patient management, even in cardiology.8
MACHINE LEARNING: TECHNIQUESML is an area of data science that forms part of what is known today as AI. ML involves the creation of systems that learn automatically; they understand by autonomously “learning” the ability to recognize complex patterns, without the need for human intervention and in datasets of any kind, including numerical, visual, and textual. As the experience of these systems increases, that is, as they are provided with new data, their performance improves to a point that may even exceed the human capacity in that task.
Although there are several ML techniques, they are usually grouped into 2 types: supervised learning and unsupervised learning.
Supervised learning techniques are undoubtedly the most widely used methods in ML and those with the best results. These procedures rely on a dataset from which the response variable to be predicted (eg, diagnosis, parameter, segmentation) is determined through the correct labeling of examples. Depending on the type of prediction, classification algorithms or regressive algorithms are used. In the first case, the aim is to identify 2 or more classes using a series of variables. In contrast, regressive algorithms seek to approximate a continuous value as much as possible.
In unsupervised learning techniques, there is no information on the variable to be predicted. These techniques must learn from the relationships among the elements of a dataset and classify them without relying on labels or categories. To do this, they look for structures, patterns, or characteristics in the source data that can be reproduced in new datasets. For this task, clustering methods are the most commonly used approaches.
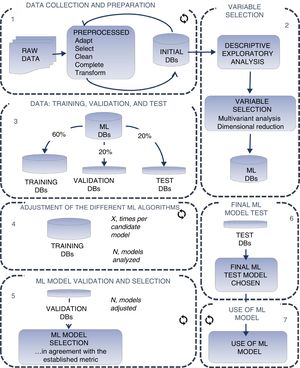
MACHINE LEARNING: METHODOLOGYThe construction of an ML model does not simply involve the application of a learning algorithm to a database, but is a whole process,9,10 which usually includes the steps shown in figure 2.
The first stages are common to conventional statistics; raw data are converted into information with structured data (preprocessed), and an initial database is constructed (step 1 of figure 2). From this database, a descriptive and exploratory analysis is performed to identify and select the most significant variables; these variables will be directly applied to the ML algorithms (step 2 of figure 2).
The next stage, now specific to ML techniques, is the division of the dataset into 3 subsets—training, validation, and testing—, typically 60%, 20%, and 20%, respectively (step 3 of figure 2).
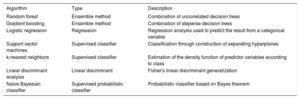
The training set is the dataset used to adjust the various ML algorithms selected (step 4 of figure 2). A wide range of classification and regression algorithms is available11 (table 1), from more classic linear techniques such as logistic regression or linear discriminant analysis to more modern ones such as aggregation-type or bagging (random forest) algorithms and packaging or boosting (xgboost) algorithms. In addition, subsampling and oversampling techniques can be used when, as often occurs in health data, one category has many more cases than another. In theory, no algorithm is better than another; its ability to make a good adjustment will depend on the characteristics of the data (eg, number of variables, linearity, normality, missing values, and continuous or categorical variables).
Most used algorithms in machine learning
| Algorithm | Type | Description |
|---|---|---|
| Random forest | Ensemble method | Combination of uncorrelated decision trees |
| Gradient boosting | Ensemble method | Combination of stepwise decision trees |
| Logistic regression | Regression | Regression analysis used to predict the result from a categorical variable |
| Support vector machines | Supervised classifier | Classification through construction of separating hyperplanes |
| k-nearest neighbors | Supervised classifier | Estimation of the density function of predictor variables according to class |
| Linear discriminant analysis | Linear discriminant | Fisher's linear discriminant generalization |
| Naive Bayesian classifier | Supervised probabilistic classifier | Probabilistic classifier based on Bayes theorem |
Once the adjustment has been made, the validation data subset is used to evaluate the quality of the model (step 5 of figure 2). To do this, the aim is to maximize the metric of greatest interest in our particular case, such as area under the ROC curve, precision, sensitivity, and accuracy (figure 3). It is common for this training-validation process to be repeated a number of times while randomizing both subsets, which is known as k-fold cross-validation. The objective is to optimize the internal parameters of the algorithm used, evaluate the robustness of the model, and determine whether the model is subadjusting or overadjusting the data, trying to find a balance between the 2 scenarios.
Once the final model has been constructed, the test data subset is used to verify that the final ML model behaves as expected with data that has not been used for its construction or validation (step 6 of figure 2). If this result differs from that obtained in the validation set, the dataset used for training is probably insufficient and should be expanded if a reliable estimator is needed before its generalized use (step 7 of figure 2).
For the implementation of ML, the most commonly used open source programming languages are currently Python and R. Both platforms can avail of libraries such as scikit-learn (Python) and caret (R) with implementations of the most widely used techniques and algorithms.
DEEP LEARNING AND CARDIAC IMAGINGImage analysis is the field with the most rapid advances in AI and is therefore highly relevant in cardiology. The daily analysis of various cardiological images can be tedious and time consuming. However, our day-to-day work can already be improved by the various tools, based on NNs, available for automatic image processing.
NNs3 are a type of AI algorithm that is analogous to the learning process occurring in the neurons of the brain. Since its development as a computational model, training algorithms and network architectures have emerged that have considerably improved learning accuracy and efficiency, functioning with ever smaller amounts of training data. When these architectures consist of numerous layers of neurons, the term DL is used. Currently, the most commonly applied NNs include convolutional NNs, recursive NNs, generative adversarial networks, and U-nets, each with different uses and architectural subtypes. NNs are also very flexible and can be used in supervised, unsupervised, and reinforcement learning contexts. For their implementation, various open source platforms are available, such as TensorFlow, Pytorch, Keras, and Caffe. The disadvantages of DL techniques include their high computational cost. In addition, a high degree of expertise is required for their correct adjustment and, in the case of supervised learning, a set of manually annotated images is required, which can sometimes be extremely expensive.
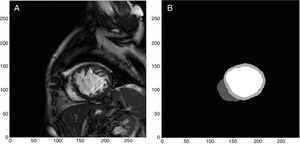
Within the field of cardiac imaging, AI techniques have several objectives. These include segmentation and identification of the different structures of the heart (figure 4),12,13 classification of images cataloged with different conditions, lesion detection and segmentation, image registration, and classification of tissues from histological images. Other tasks are related to the generation of artificial images that are as realistic as possible.
Example of automatic segmentation and identification of the left and right ventricle through deep learning performed in our department from images obtained with a 1.5-T Philips Achieva resonance system.12 From original images (A), the free neural network13 was able to identify and segment the left and right ventricles (B, white color for the left ventricle, light gray color for the myocardium of the left ventricle, and dark gray color for the right ventricle).
Research applying DL techniques to cardiological imaging has burgeoned in the last 3 years. However, the use of DL techniques in medical imaging has made more of a mark in other disciplines, such as neurology and pneumology.14 Among the challenges likely to be explored in the coming years are combined analyses of different imaging sources, the incorporation of clinical data and medical reports, and the study of temporal changes in cardiac images.
LIMITATIONS OF MACHINE LEARNING AND DEEP LEARNINGThe main characteristic of AI in terms of ML or DL models is that learning is based on the identification of patterns in datasets. This philosophy is simultaneously a strong point, because computers are extremely efficient and precise at finding such patterns when they exist, and a limitation, for several reasons. First, because the amount of data required to obtain an accurate model can be substantial. This can be a problem in medicine, where the implementation of automatic data collection systems is just beginning, such systems must meet established legal and ethical criteria, and some rare diseases inevitably have few studies. In addition, even with algorithms with good results for a dataset, ML and DL models suffer from an inability to correctly detect and classify cases that they have not previously seen. Along these lines, the reliability and quality of the data source are essential for an algorithm to be realistic and correct. If the dataset used to adjust the model is itself biased, the model may not be well generalized to other populations.15 This problem is nothing new in our daily work because we accept risk scales based on data from other populations, such as the Framingham score for cardiovascular risk, resulting in erroneous results that overestimate or underestimate the risk upon application to other populations.16 Initiatives are underway to standardize the requirements that must be met by an AI algorithm for its possible practical application.17 For all of these reasons, it seems clear that particular effort will be needed for the integration of datasets from different populations.
Another important limitation is the opacity and interpretability of the ML models, particularly the DL models. These techniques are used as “black boxes”, which are fed inputs to obtain an output, namely a prediction. Thus, ML offers us answers in the form of predictions, but not a biological explanation. Although there are various methodologies to interpret the results of a model and verify its correct functioning through analysis of the specific weights of parameters or variables and to highlight the most discriminating parts or the individual explanation of each prediction,18 it is not currently possible to know exactly why the more complex ML and DL models make a certain decision. This limitation makes it difficult to generate knowledge and to apply AI to an area as critical as medicine.
MACHINE LEARNING AND CLASSIC STATISTICS: SIMILARITIES AND DIFFERENCESAlthough these 2 specialties have different origins, they also have common features, despite their distinct purposes.
The cornerstone of classic statistics is inference. In inference, the observations in a sample are extended to the entire population, generally creating a mathematical model that defines the relationship among variables. The strength of ML lies in a prediction based on the available information, without the need to know the mechanisms relating the variables to each other. These characteristics are not exclusive because, to a greater or lesser extent, both disciplines use inferential techniques to improve their results (ML) or predictive algorithms to corroborate inferential conclusions (statistics).
Compared with statistical techniques, those of ML do not require prior suppositions about the variables, have the ability to handle cases with missing data, and improve their reliability when large volumes of data are available by detecting complex relationships among variables.
LEGAL AND ETHICAL ASPECTS OF ARTIFICIAL INTELLIGENCEWhen AI is applied to the world of medicine, issues can arise that are not strictly related to the quality of the ML and DL algorithms. These are connected to legal problems associated with the automatic processing of personal data or how to apply ML in day-to-day practice.
Practically all AI techniques studied to date require a certain amount of data for the training and validation of predictive models. In our case, the information used is particularly sensitive because it generally involves patients’ personal and clinical data. Therefore, the proper use of this information from a legal point of view is a critical aspect.
Personal data protection is a fundamental right enshrined in the Spanish Constitution. However, such data may be used under certain conditions if a service is being provided to society. If both the organization collecting the information and that processing the data are fiscally resident in Spain, they must, since 2018, comply with Organic Law 3/2018 of December 5th, 2018, on the Protection of Personal Data and the Guarantee of Digital Rights.19 According to this legislation, the use of personal data is allowed for statistical purposes, as long as the results are aggregated. It is also necessary to adopt measures to anonymize and protect these data, and a number of rights of the individuals concerned is recognized, such as to be informed, have access, and be allowed rectification. Therefore, given that the use of cloud computing services is very common in AI due to their flexibility and greater computing power, the largest providers have adapted their data-processing policies to provide a completely secure service. In addition, these companies adhere to the CISPE (Cloud Infrastructure Services Providers in Europe) Code of Conduct to guarantee that their data protection standards comply with the legislation. Likewise, in situations where an AI model is used as a diagnostic or decision-making tool, the patient must be informed and provide consent.
The subtle discrimination inherent in the provision of health services can also be a problem when AI models are being developed and applied.15 For example, age is considered when scarce resources are being rationed, such as heart transplants. Such a consideration can lead to self-fulfilling prophecies: if physicians withdraw care for patients due to their advanced age, ML systems may conclude that the care of older patients is always fatal. On the other hand, it is also possible for ML models to help to resolve disparities in the provision of medical care if algorithms can be constructed to compensate for known biases or identify pressing areas of research.
There is broad discussion about how AI will affect the workflow of medical staff. AI might not eliminate jobs, but rather displace medical practice tasks. Routine and tiring jobs could be performed by machines to free up time and allow medical professionals to carry out more complex and sensitive tasks. However, the use of automatic diagnostic tools can lead to problems of bias in decision-making and in the assignment of responsibility in the case of error. Physicians may be inclined to review a diagnostic test in a more relaxed way if an algorithm has previously returned a negative result. The design of decision-making systems that involve both machines and humans is a crucial aspect for AI, and there are intermediate models between conventional clinical practice and fully automated systems, each with different characteristics of cost and time efficiency, risk, and interpretability.20 The implementation of these systems will be an area with a fundamental role for clinicians as validators.
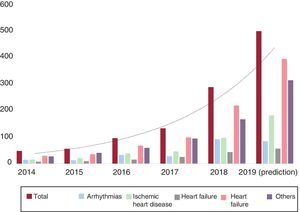
ARTIFICIAL INTELLIGENCE IN CARDIOLOGYExamples of AI using ML or DL are becoming more and more common in cardiology.21 This section presents the evolution of the contributions of AI to the different application areas of cardiology (figure 5).
Temporal changes in the publications indexed in PubMed on artificial intelligence, machine learning, and deep learning according to the area of interest in cardiology. The details of the publications by area of interest and the search methodology are described in the supplementary material.
The supplementary material for this article includes a list of the contributions of the techniques described in several areas of cardiology in the last 5 full years and from January to March 2019, as well as a detailed description of the publications that we consider of most interest and that will help to explain the ML and DL models (table 2).22–40 Many of the publications reviewed are the result of common initiatives in AI, such as challenges promoted in conferences24,41 and datathons. In datathons, which are public and open competitions, people interested in the subject, from beginners to experts, work together with common databases to achieve an established goal. Participants must find a solution to a defined objective in the datasets provided. The most accurate and creative solutions are awarded a prize and made publicly accessible.
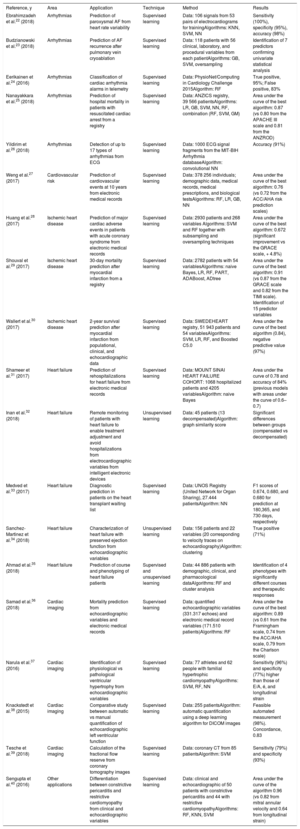
Relevant contributions of artificial intelligence to the various application areas of cardiology
| Reference, y | Area | Application | Technique | Method | Results |
|---|---|---|---|---|---|
| Ebrahimzadeh et al.22 (2018) | Arrhythmias | Prediction of paroxysmal AF from heart rate variability | Supervised learning | Data: 106 signals from 53 pairs of electrocardiograms for trainingAlgorithms: KNN, SVM, NN | Sensitivity (100%), specificity (95%), accuracy (98%) |
| Budzianowski et al.23 (2018) | Arrhythmias | Prediction of AF recurrence after pulmonary vein cryoablation | Supervised learning | Data: 118 patients with 56 clinical, laboratory, and procedural variables from each patientAlgorithms: GB, SVM, oversampling | Identification of 7 predictors confirming univariate statistical analysis |
| Eerikainen et al.24 (2016) | Arrhythmias | Classification of cardiac arrhythmia alarms in telemetry | Supervised learning | Data: PhysioNet/Computing in Cardiology Challenge 2015Algorithm: RF | True positive, 95%; False positive, 83% |
| Nanayakkara et al.25 (2018) | Arrhythmias | Prediction of hospital mortality in patients with resuscitated cardiac arrest from a registry | Supervised learning | Data: ANZICS registry, 39 566 patientsAlgorithms: LR, GB, SVM, NN, RF, combination (RF, SVM, GM) | Area under the curve of the best algorithm: 0.87 (vs 0.80 from the APACHE III scale and 0.81 from the ANZROD) |
| Yildirim et al.26 (2018) | Arrhythmias | Detection of up to 17 types of arrhythmias from ECG | Supervised learning | Data: 1000 ECG signal fragments from the MIT-BIH Arrhythmia databaseAlgorithm: convolutional NN | Accuracy (91%) |
| Weng et al.27 (2017) | Cardiovascular risk | Prediction of cardiovascular events at 10 years from electronic medical records | Supervised learning | Data: 378 256 individuals; demographic data, medical records, medical prescriptions, and biological testsAlgorithms: RF, LR, GB, NN | Area under the curve of the best algorithm: 0.76 (vs 0.72 from the ACC/AHA risk prediction scales) |
| Huang et al.28 (2017) | Ischemic heart disease | Prediction of major cardiac adverse events in patients with acute coronary syndrome from electronic medical records | Supervised learning | Data: 2930 patients and 268 variables Algorithms: SVM and RF together with subsampling and oversampling techniques | Area under the curve of the best algorithm: 0.672 (significant improvement vs the GRACE scale, + 4.8%) |
| Shouval et al.29 (2017) | Ischemic heart disease | 30-day mortality prediction after myocardial infarction from a registry | Supervised learning | Data: 2782 patients with 54 variablesAlgorithms: naive Bayes, LR, RF, PART, ADABoost, ADtree | Area under the curve of the best algorithm: 0.91 (vs 0.87 from the GRACE scale and 0.82 from the TIMI scale). Identification of 15 predictor variables |
| Wallert et al.30 (2017) | Ischemic heart disease | 2-year survival prediction after myocardial infarction from populational, clinical, and echocardiographic data | Supervised learning | Data: SWEDEHEART registry, 51 943 patients and 54 variablesAlgorithms: SVM, LR, RF, and Boosted C5.0 | Area under the curve of the best algorithm (0.84), negative predictive value (97%) |
| Shameer et al.31 (2017) | Heart failure | Prediction of rehospitalizations for heart failure from electronic medical records | Supervised learning | Data: MOUNT SINAI HEART FAILURE COHORT: 1068 hospitalized patients and 4205 variablesAlgorithm: naive Bayes | Area under the curve of 0.78 and accuracy of 84% (previous models with areas under the curve of 0.6–0.7) |
| Inan et al.32 (2018) | Heart failure | Remote monitoring of patients with heart failure to enable treatment adjustment and avoid hospitalizations from electrocardiographic variables from intelligent electronic devices | Unsupervised learning | Data: 45 patients (13 decompensated)Algorithm: graph similarity score | Significant differences between groups (compensated vs decompensated) |
| Medved et al.33 (2017) | Heart failure | Diagnostic prediction in patients on the heart transplant waiting list | Supervised learning | Data: UNOS Registry (United Network for Organ Sharing), 27.444 patientsAlgorithm: NN | F1 scores of 0.674, 0.680, and 0.680 for prediction at 180,365, and 730 days, respectively |
| Sanchez-Martinez et al.34 (2018) | Heart failure | Characterization of heart failure with preserved ejection function from echocardiographic variables | Unsupervised learning | Data: 156 patients and 22 variables (20 corresponding to velocity traces on echocardiography)Algorithm: clustering | True positive (71%) |
| Ahmad et al.35 (2018) | Heart failure | Prediction of course and phenotyping of heart failure patients | Supervised and unsupervised learning | Data: 44 886 patients with demographic, clinical, and pharmacological dataAlgorithms: RF and cluster analysis | Identification of 4 phenotypes with significantly different courses and therapeutic responses |
| Samad et al.36 (2018) | Cardiac imaging | Mortality prediction from echocardiographic variables and electronic medical records | Supervised learning | Data: quantified echocardiographic variables (331.317 echoes) and electronic medical record variables (171.510 patients)Algorithms: RF | Area under the curve of the best algorithm: 0.89 (vs 0.61 from the Framingham scale, 0.74 from the ACC/AHA scale, 0.79 from the Charlson scale) |
| Narula et al.37 (2016) | Cardiac imaging | Identification of physiological vs pathological ventricular hypertrophy from echocardiographic variables | Supervised learning | Data: 77 athletes and 62 people with familial hypertrophic cardiomyopathyAlgorithms: SVM, RF, NN | Sensitivity (96%) and specificity (77%) higher than those of E/A, é, and longitudinal strain |
| Knackstedt et al.38 (2015) | Cardiac imaging | Comparative study between automatic vs manual quantification of echocardiographic left ventricular function | Supervised learning | Data: 255 patientsAlgorithm: automatic quantification using a deep learning algorithm for DICOM images | Feasible automated measurement (98%). Concordance, 0.83 |
| Tesche et al.39 (2018) | Cardiac imaging | Calculation of the fractional flow reserve from coronary tomography images | Supervised learning | Data: coronary CT from 85 patientsAlgorithm: SVM | Sensitivity (79%) and specificity (93%) |
| Sengupta et al.40 (2016) | Other applications | Differentiation between constrictive pericarditis and restrictive cardiomyopathy from clinical and echocardiographic variables | Supervised learning | Data: clinical and echocardiographic of 50 patients with constrictive pericarditis and 44 with restrictive cardiomyopathyAlgorithms: RF, KNN, SVM | Area under the curve of the algorithm 0.96 (vs 0.82 from mitral annular velocity and 0.64 from longitudinal strain) |
ACC/AHA, American College of Cardiology/American Heart Association; AF, atrial fibrillation; ECG, electrocardiography; GB, gradient boosting; KNN, k-nearest neighbors; LR, logistic regression; NN, neural network; RF, random forest; SVMs, support vector machines.
One of the most widespread applications of ML in cardiology is the prediction of cardiac arrhythmias. Numerous studies address predictive models for atrial fibrillation development, including paroxysmal, due to its impact and clinical implications, using supervised learning with predictive ML systems composed of different subprocesses: signal preprocessing, extraction of significant variables, and classification algorithms.22,42 Similar ML models have been developed to improve telemonitoring alarm management24 and to predict the occurrence of ventricular arrhythmias,43,44 the response to invasive ablation procedures such as cryoablation,2,3 and even mortality after resuscitated cardiac arrest.25 DL techniques have been used with great success to detect distinct types of arrhythmias through direct analysis of images or electrocardiographic signals.26 Another application has been to use unsupervised learning to identify phenotypes to classify hypertrophic cardiomyopathies with different arrhythmic risk.45
Ischemic heart diseaseAI has been applied to standard electronic medical records from primary care to predict the risk of cardiovascular disease in the general population and has been shown to be superior to the traditionally used risk scales.27 In addition, supervised learning techniques have been applied to the prognostic prediction of stable ischemic heart disease,46 coronary syndrome,28 and mortality of patients with myocardial infarction by analyzing the results of individual hospitals29 or large registries such as SWEDEHEART.30 The results are disparate due to the sizes of the samples, which shows that ML techniques obtain better results with large sample sizes.
Heart failureML systems could allow the optimization of avoidable hospitalizations due to heart failure by more accurately identifying patients susceptible to cardiac decompensation after hospital discharge than classic risk scales31,47; these results contradict initial experience in this field48 and highlight the need to adjust the methodologies of the systems.49 In addition, initial work has addressed the usefulness of AI as a management system in the telemonitoring of patients with HF. Studies have shown that ML is feasible50 and that it might improve the clinical course of these patients.32 Another important area is heart transplant, with ML systems applied to predict the probability of death or transplant in patients on the waiting list or transplant success.33 Notably, the clinical response to cardiac resynchronization can be predicted using ML systems.51
An excellent example of a combination of ML and DL methodologies in this field is the prediction of diastolic dysfunction through analysis of echocardiographic data.52 This research, along with other examples that have used unsupervised learning,34,53 shows that these procedures can facilitate the standardization and interpretation of complex heart diseases, such as the diagnosis of heart failure with preserved systolic function, enhancing decision-making.
Another study conducted with unsupervised techniques and a large amount of data from the Swedish Heart Failure Registry was able to identify 4 phenotypes with different clinical courses and therapeutic responses.35
Cardiac imagingExamples of AI using imaging data are beginning to become popular and will be responsible for a new revolution in the world of cardiac imaging. ML techniques using data generated from cardiac imaging quantification have been successfully used to, for example, predict cardiovascular mortality from extensive echocardiographic databases.36 Similar ML models have been developed to differentiate the echocardiographic patterns of the physiological ventricular hypertrophy typical of athletes from the findings of familial hypertrophic cardiomyopathy.37
Apart from the ML methods, DL has been used to directly analyze images in several different application domains. Most of the efforts have focused on segmentation tasks for cardiac tissue and anatomical structures (eg, the endocardium; figure 4), which is usually the previous step in other study types, such as those involving injury detection or disease classification. The first study purely conducted with DL dates from 2013 and involved segmentation of the left ventricle from echocardiography.54 Since 2009, datathon-style contests have systematically used a cardiac imaging tool, mainly with the objective of left and right ventricular segmentation and based on different images, typically magnetic resonance imaging and computed tomography.14,41,55 In recent years, there have been repeated publications on NN architectures that have improved the state of the technique in terms of heart segmentation, in both 2-dimensions56,57 and 3-dimensions.41,58,59 There have even been comparative scientific studies of the automated determination of the endocardium and calculation of the left ventricular ejection fraction from DL algorithms directly applied to DICOM images vs manual tracing, with excellent concordance and speed.38
In the field of echocardiographic imaging, advances have already been made to implement a fully automatic interpretation, through the identification of viewpoints, image segmentation, the quantification of structures and functions, and disease detection.60
Other results related to nonsegmentation tasks obtained in recent years include calculation of the fractional flow reserve from coronary computed tomography images,39 measurement of calcium in coronary arteries,61 quantification and characterization of coronary and carotid artery tissues,62,63 improvement or generation of cardiac images,64 detection of stenosis and atherosclerosis,65 and differentiation of constrictive pericarditis from restrictive cardiomyopathy.40
Other applicationsAI is being widely used in other application domains, which reflects its versatility. The search for patterns encompasses a multitude of functionalities, from the prediction of cardiovascular risk through fundus analysis4 to that of acute renal failure after cardiac surgery, for example.66 These patterns can better identify groups of patients with dissimilar risk, and their incorporation into clinical practice can help to eliminate uncertainties and improve clinical outcomes.
CONCLUSIONSAlthough AI is often viewed as a futuristic and distant concept, the truth is that this technology is already in use in all types of areas, including cardiology. Due to the digitization of large amounts of data, the development of ML algorithms, and improvements in computer power in recent decades, AI can provide excellent opportunities for task automation, the application of precision medicine, or research progress through the detection of complex patterns in medical databases.67 A particular case is that of medical image analysis, with DL techniques having undergone a true revolution and their application to the area of cardiology already yielding excellent results. However, there is still a long way to go before these techniques can be widely applied to clinical practice. Essential prerequisites are large databases with high-quality information and the evaluation and integration of AI into realistic clinical contexts; therefore, the understanding of AI and its applications in our field is essential for the present and future development of cardiology.
FUNDINGThis work was funded by the Carlos III Health Institute (Ministry of Science, Innovation, and Universities) within the CIBERCV, through which P.I. Dorado-Díaz and J. Sampedro-Gómez were hired.
CONFLICTS OF INTERESTV. Vicente-Palacios works for Philips Healthcare Ibérica. The remaining researchers have no conflicts of interest to declare.
The authors of this article would like to thank Antonio Sánchez, Rafael Vidal, Manuel Jiménez-Navarro, and Purificación Galindo for reviewing the manuscript prior to its submission to Revista Española de Cardiología. Their comments and advice were very helpful and no doubt contributed to the drafting of this article.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.05.014