Patients with a single syncopal episode (SSE) and complete bundle branch block (cBBB) are frequently managed more conservatively than patients with recurrent episodes (RSE). The objective of this study was to analyze if there are differences between patients with single or recurrent unexplained syncope and cBBB in arrhythmic risk, the diagnostic yield of tests, and clinical outcomes.
MethodsCohort study of consecutive patients with unexplained syncope and cBBB with a median follow-up time of 3 years. The patients were evaluated via a stepwise workup protocol based on electrophysiological study (EPS) and long-term follow-up with an implantable cardiac monitor.
ResultsOf the 503 patients included in the study, 238 (47.3%) had had only 1 syncopal episode. The risk of an arrhythmic syncope was similar in both groups (58.8% in SSE vs 57.0% in RSE; P=.68), also after adjustment for possible confounding variables (HR, 1.06; 95%CI, 0.81-1.38; P=.674). No significant differences between the groups were found in the EPS results and implantable cardiac monitor diagnostic yield. A total of 141 (59.2%) patients with SSE and 154 (58.1%) patients with RSE required cardiac device implantation (P=.797). After appropriate treatment, 35 (7%) patients had recurrence of syncope. The recurrence rate and mortality were also similar in both groups.
ConclusionsPatients with cBBB and unexplained syncope are at high risk of an arrhythmic etiology, even after the first syncopal episode. Patients with SSE and RSE have a similar arrhythmic risk and similar outcomes, and therefore there is no clinical justification for not managing them in the same manner.
Keywords
Arrhythmia, specifically paroxysmal advanced atrioventricular block (aAVB), is the most common cause of unexplained syncope in patients with complete bundle branch block (cBBB).1,2 However, up to nearly 40% of cases may be due to a nonarrhythmic cause.1,3,4 Clinical practice guidelines recommend either systematic study of the potential cause (including an electrophysiological study [EPS] and the implantation of a cardiac monitor [ICM]) or empirical pacemaker implantation in patients with syncope and cBBB.1,5 In this regard, a clinical history of previous syncope, or having recurrent syncopal episodes, are conditions not specifically considered in these recommendations or included in the most commonly used syncope risk scores.1,6,7 Nevertheless, in the clinical setting, patients with a first syncopal episode are frequently managed conservatively and are discharged without a complete assessment or specific treatment.8.9 This may be due to the assumption by some clinicians that the risk of major adverse events is lower in patients with a first syncope than in those with recurrent syncope. For example, in a recent EHRA survey, 79% of physicians responded that they implant an ICM in high-risk patients with recurrent syncope, but there is no reference to patients presenting with their first episode.8 In addition, only 67% of them considered carrying out an EPS in patients with syncope and inconclusive noninvasive testing in the presence of bifascicular block. Moreover, in a recent study aiming to analyze the diagnostic and therapeutic strategies used in patients with syncope and cBBB, only those patients with recurrent syncope were eligible for the study.10
Few studies have investigated the arrhythmic risk and outcomes of recurrent syncopal episodes, and some of their results are contradictory. Furthermore, as far as we know, no previous studies have specifically evaluated the potential increase in arrhythmic risk in patients with unexplained syncope and cBBB depending on whether it is an isolated or recurrent episode. We hypothesizes that patients presenting their first syncopal episode would have a similar arrhythmic risk to patients with recurrent episodes, and therefore, there should be no differences in their management.
The aim of this study was to analyze potential differences in the arrhythmic risk, diagnostic yield of testing, and clinical outcomes in patients with single vs recurrent unexplained syncope and cBBB.
METHODSStudy populationProspective observational study of a consecutive patient cohort at a tertiary referral hospital (Hospital Universitari Vall D’Hebron, Barcelona, Spain). From January 2010 to October 2021, we included patients admitted for syncope with cBBB, in whom no definitive diagnosis was reached for the syncope in the initial assessment in the emergency department. We excluded patients younger than 18 years, those with pacemakers or implantable cardiac defibrillators in situ, patients with left ventricular ejection fraction<35% or with another direct indication for implantable cardiac defibrillator, those with severe comorbidities making it impracticable to undergo the study protocol (such us patients with less than 1 year of life expectancy or completely dependent for basic activities of daily living), and those who withheld informed consent for some of the tests included in the workup. In May 2022, we collected the final follow-up data of the patients. The patients’ clinical details, syncope characteristics, therapeutic management, and follow-up were recorded at the time of hospital admission. Some of the patients included in this article (n=443) had been previously included in a study intended to assess sex-related differences in this population.4
The study complies with the Declaration of Helsinki and was approved by the local ethics committee.
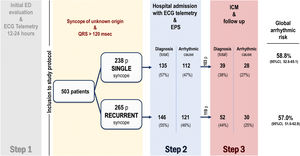
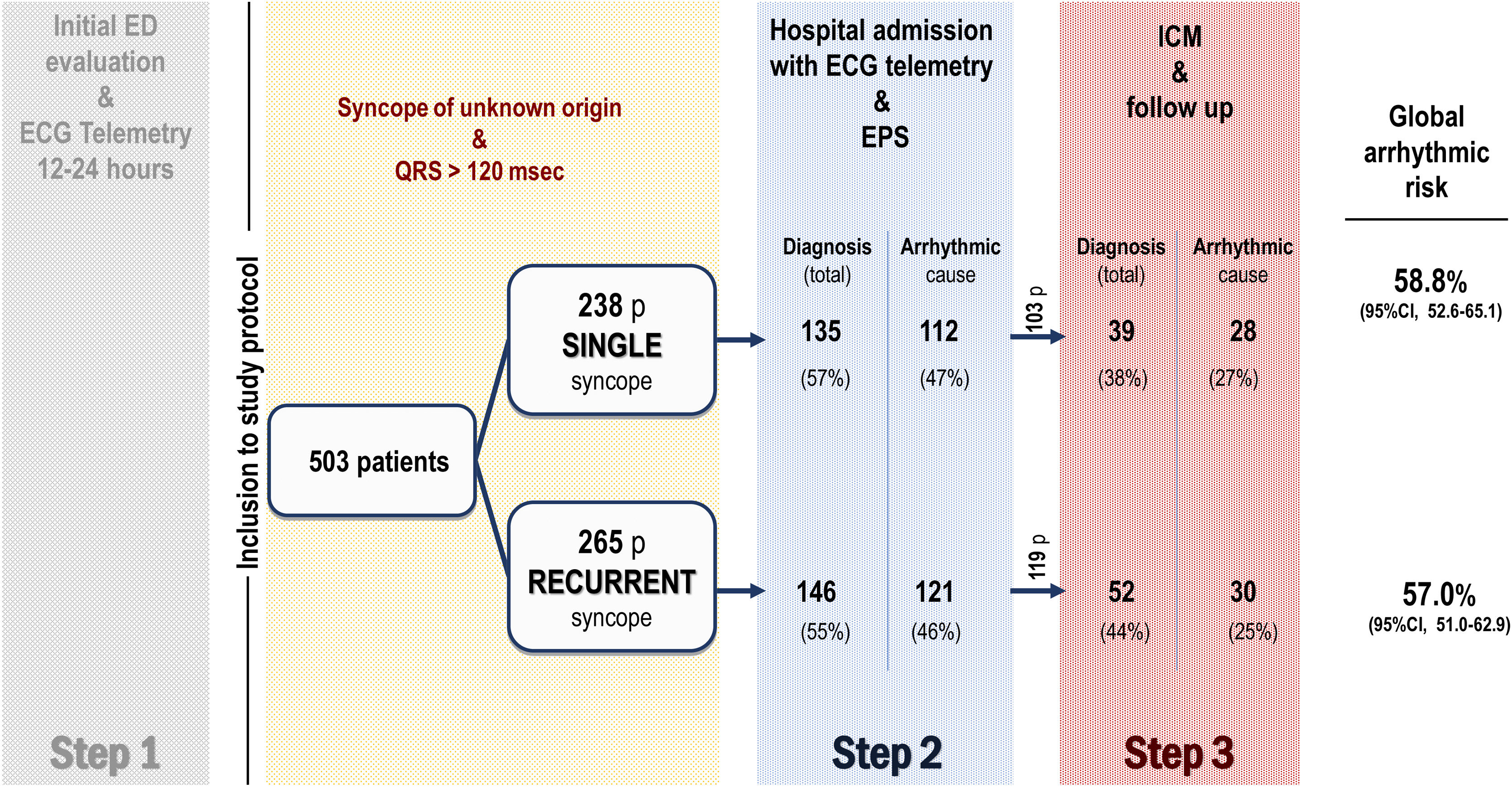
Study protocolPatients were systematically managed according to the local clinical protocol,4 which is based on recommendations of the European Society of Cardiology (ESC) syncope guidelines.1,2 Briefly, the clinical diagnostic protocol applied in the study is based on 3 phases or steps. Step 1, prior to the patients’ inclusion in the study, consists of the initial assessment in the emergency department. Those cases with no certain or highly probable diagnosis are then considered as having unexplained syncope, and these patients are admitted to the hospital with continuous electrocardiogram (ECG) monitoring. Step 2 involves hospital admission with continuous ECG monitoring and an invasive EPS. Step 3 involves implanting an ICM with subsequent clinical monitoring (figure 1). The syncope was treated according to the clinical practice guidelines in line with the confirmed etiology.1 After hospital discharge, patients were followed up in the outpatient cardiology clinic, and those who had received a cardiac device were also followed up with the corresponding remote function.
More detailed information on the diagnostic protocol, EPS, ICM monitoring, and treatment are provided in the .
Definitions and endpointsThe main etiological mechanism of the syncope was established as certain or highly probable according to the definitions included in the ESC guidelines on syncope1 (). Syncope due to aAVB or severe conduction disturbances (sCD), sinus node dysfunction, fast supraventricular tachycardia or ventricular tachycardia (VT) where considered an arrhythmic syncope. The patient details were analyzed by 2 cardiologists specialized in syncope to establish the definitive diagnosis according to the definitions. The etiology of syncopal recurrences was defined in the same manner.
The endpoints of the study were the risk of an arrhythmic syncope, test diagnostic yields, need for cardiac pacing related to syncope, and syncope recurrences after treatment and mortality.
Statistical analysisCategorical variables are presented as absolute numbers (No.) and percentages. Continuous quantitative variables are presented as median and interquartile ranges [IQR]. The comparison of numerical variables was performed using the Student t test or Wilcoxon rank-sum test, depending on the distribution of the variables. The chi-square test or Fisher exact test was used to compare qualitative variables as appropriate. The Wald method was used to calculate the confidence interval for the population rates and proportions. Survival functions were estimated using the Kaplan-Meier method and their comparison was performed by the log-rank test. A Manten-Hazel test was used to evaluate the linear relation between the number of previous syncopal episodes and arrhythmic risk. A Cox proportional hazards multivariate model was developed to assess the association between previous syncopal episodes and arrhythmic syncope and to adjust for possible confounder variables. When we estimated the Cox proportional hazards model, we checked the different possible interactions between pairs of explanatory variables and found no statistically significant results. A saturated model including all clinically relevant covariates1,2,4,11–19 was estimated, and simplified models were evaluated. A relevant confounder effect was considered to be present when the hazard ratios (HRs) with and without the adjustment for the potential confounder differed by more than 10%. The most precise model with all relevant clinical covariates was finally selected. A P<.05 was considered statistically significant for all tests. All the statistical analyses were performed using Stata, version 15.1.0 (StataCorp LLC College Station, United States).
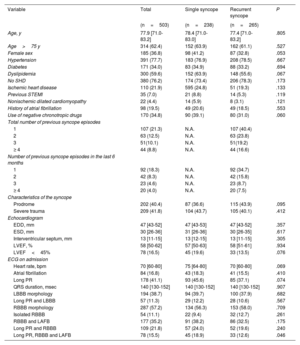
RESULTSStudy populationA total of 503 patients were included in the study, 265 (52.7%) with recurrent syncope (recurrent syncope group [RSG]) and 238 (47.3%) without previous syncopal episodes (single syncope group [SSG]) before the index event. Baseline characteristics are shown in table 1. Median age was 77.9 years [IQR: 71.0-83.2] and 36.8% where women. No relevant clinical differences were observed between the groups. In the RSG, 40.4% had had 1 previous syncope, while 16.6% reported 4 or more previous syncopal episodes.
Baseline characteristics of patients included in the study
| Variable | Total | Single syncope | Recurrent syncope | P |
|---|---|---|---|---|
| (n=503) | (n=238) | (n=265) | ||
| Age, y | 77.9 [71.0-83.2] | 78.4 [71.0-83.0] | 77.4 [71.0-83.2] | .805 |
| Age>75 y | 314 (62.4) | 152 (63.9) | 162 (61.1) | .527 |
| Female sex | 185 (36.8) | 98 (41.2) | 87 (32.8) | .053 |
| Hypertension | 391 (77.7) | 183 (76.9) | 208 (78.5) | .667 |
| Diabetes | 171 (34.0) | 83 (34.9) | 88 (33.2) | .694 |
| Dyslipidemia | 300 (59.6) | 152 (63.9) | 148 (55.6) | .067 |
| No SHD | 380 (76.2) | 174 (73.4) | 206 (78.3) | .173 |
| Ischemic heart disease | 110 (21.9) | 595 (24.8) | 51 (19.3) | .133 |
| Previous STEMI | 35 (7.0) | 21 (8.8) | 14 (5.3) | .119 |
| Nonischemic dilated cardiomyopathy | 22 (4.4) | 14 (5.9) | 8 (3.1) | .121 |
| History of atrial fibrillation | 98 (19.5) | 49 (20.6) | 49 (18.5) | .553 |
| Use of negative chronotropic drugs | 170 (34.8) | 90 (39.1) | 80 (31.0) | .060 |
| Total number of previous syncope episodes | ||||
| 1 | 107 (21.3) | N.A. | 107 (40.4) | |
| 2 | 63 (12.5) | N.A. | 63 (23.8) | |
| 3 | 51(10.1) | N.A. | 51(19.2) | |
| ≥ 4 | 44 (8.8) | N.A. | 44 (16.6) | |
| Number of previous syncope episodes in the last 6 months | ||||
| 1 | 92 (18.3) | N.A. | 92 (34.7) | |
| 2 | 42 (8.3) | N.A. | 42 (15.8) | |
| 3 | 23 (4.6) | N.A. | 23 (8.7) | |
| ≥ 4 | 20 (4.0) | N.A. | 20 (7.5) | |
| Characteristics of the syncope | ||||
| Prodrome | 202 (40.4) | 87 (36.6) | 115 (43.9) | .095 |
| Severe trauma | 209 (41.8) | 104 (43.7) | 105 (40.1) | .412 |
| Echocardiogram | ||||
| EDD, mm | 47 [43-52] | 47 [43-53] | 47 [43-52] | .357 |
| ESD, mm | 30 [26-36] | 31 [26-36] | 30 [26-35] | .617 |
| Interventricular septum, mm | 13 [11-15] | 13 [12-15] | 13 [11-15] | .305 |
| LVEF, % | 58 [50-62] | 57 [50-63] | 58 [51-61] | .934 |
| LVEF<45% | 78 (16.5) | 45 (19.6) | 33 (13.5) | .076 |
| ECG on admission | ||||
| Heart rate, bpm | 70 [60-80] | 75 [64-80] | 70 [60-80] | .069 |
| Atrial fibrillation | 84 (16.8) | 43 (18.3) | 41 (15.5) | .410 |
| Long PR | 178 (41.1) | 93 (45.6) | 85 (37.1) | .074 |
| QRS duration, msec | 140 [130-152] | 140 [130-152] | 140 [130-152] | .907 |
| LBBB morphology | 194 (38.7) | 94 (39.7) | 100 (37.9) | .682 |
| Long PR and LBBB | 57 (11.3) | 29 (12.2) | 28 (10.6) | .567 |
| RBBB morphology | 287 (57.2) | 134 (56.3) | 153 (58.0) | .709 |
| Isolated RBBB | 54 (11.1) | 22 (9.4) | 32 (12.7) | .261 |
| RBBB and LAFB | 177 (35.2) | 91 (38.2) | 86 (32.5) | .175 |
| Long PR and RBBB | 109 (21.8) | 57 (24.0) | 52 (19.6) | .240 |
| Long PR, RBBB and LAFB | 78 (15.5) | 45 (18.9) | 33 (12.6) | .046 |
bpm, beats per minute; EDD, end-diastolic diameter; ESD, end-systolic diameter; LAFB, left anterior fascicular block; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; mm, millimeters; msec, milliseconds; NA, not applicable; RBBB, right bundle branch block; SHD, structural heart disease; STEMI, ST elevated myocardial infarction.
Values are expressed as No. (%). Quantitative variables are expressed as median [interquartile range].
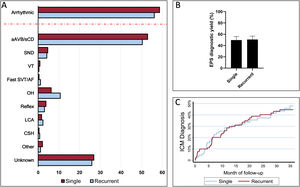
Figure 1 summarizes the study flow chart. A definitive or highly probable diagnosis of the main cause of syncope was reached in 372 patients (74%) (73.1% in SSG and 74.3% in RSG, P=.754). In 281 (55.9%) patients, the diagnosis was reached in step 2 (in 252 patients after a positive EPS and in another 29 after showing symptoms with diagnostic criteria during the hospital stay). In step 3, a definitive diagnosis was reached in an additional 91 (18.1%) patients (80 due to the ICM findings and 11 due to clinical criteria). Figure 2A shows the diagnosis of the main causes of syncope. No significant differences were found between the 2 groups. Detailed information on the diagnosis in each step is provided in .
Etiological diagnosis and diagnostic test yields. A: etiological diagnosis reached by the group. The dashed red line separates the overall arrhythmia diagnosis from the specific etiologies. B: electrophysiological study diagnostic yield by groups. C: ICM cumulative diagnostic yield according to time of follow-up (Kaplan-Meier failure estimates curve). aAVB/sCD, advanced atrioventricular block or severe conduction disturbances; AF, atrial fibrillation; CSH, carotid sinus hypersensitivity; EPS, electrophysiological study; ICM, implantable cardiac monitor; LCA, low cardiac output; OH, orthostatic hypotension; SND, sinus node dysfunction; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
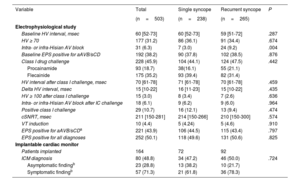
The main findings in the EPS and in the ICM are shown in table 2. EPS had a similar diagnostic yield in both groups (49.6% in SSG vs 50.6% in RSG, P=.825) (figure 2B) and a similar negative predictive value for arrhythmic syncope (74.2% [95%CI, 65.7-81.2] in SSG vs 77.1% [95%CI, 69.2-83.5] in RSG). The diagnostic yield of ICM was also similar between the 2 groups (47.2% in SGG vs 50.0% in RSG, P=.724) (figure 2C).
Electrophysiological study and implantable cardiac monitor
| Variable | Total | Single syncope | Recurrent syncope | P |
|---|---|---|---|---|
| (n=503) | (n=238) | (n=265) | ||
| Electrophysiological study | ||||
| Baseline HV interval, msec | 60 [52-73] | 60 [52-73] | 59 [51-72] | .287 |
| HV ≥ 70 | 177 (31.2) | 86 (36.1) | 91 (34.4) | .674 |
| Intra- or infra-Hisian AV block | 31 (6.3) | 7 (3.0) | 24 (9.2) | .004 |
| Baseline EPS positive for aAVB/sCD | 192 (38.2) | 90 (37.8) | 102 (38.5) | .876 |
| Class I drug challenge | 228 (45.9) | 104 (44.1) | 124 (47.5) | .442 |
| Procainamide | 93 (18.7) | 38(16.1) | 55 (21.1) | |
| Flecainide | 175 (35.2) | 93 (39.4) | 82 (31.4) | |
| HV interval after class I challenge, msec | 70 [61-78] | 71 [61-78] | 70 [61-78] | .459 |
| Delta HV interval, msec | 15 [10-22] | 16 [11-23] | 15 [10-22] | .435 |
| HV ≥ 100 after class I challenge | 15 (3.0) | 8 (3.4) | 7 (2.6) | .636 |
| Intra- or infra-Hisian AV block after IC challenge | 18 (6.1) | 9 (6.2) | 9 (6.0) | .964 |
| Positive class I challenge | 29 (10.7) | 16 (12.1) | 13 (9.4) | .474 |
| cSNRT, msec | 211 [150-281] | 214 [150-266] | 210 [150-300] | .574 |
| VT induction | 10 (4.4) | 5 (4.24) | 5 (4.6) | .910 |
| EPS positive for aAVB/sCDa | 221 (43.9) | 106 (44.5) | 115 (43.4) | .797 |
| EPS positive for all diagnoses | 252 (50.1) | 118 (49.6) | 131 (50.6) | .825 |
| Implantable cardiac monitor | ||||
| Patients implanted | 164 | 72 | 92 | |
| ICM diagnosis | 80 (48.8) | 34 (47.2) | 46 (50.0) | .724 |
| Asymptomatic findingb | 23 (28.8) | 13 (38.2) | 10 (21.7) | |
| Symptomatic findingb | 57 (71.3) | 21 (61.8) | 36 (78.3) |
aAVB/sCD, advanced atrioventricular block or severe conduction disturbances; cSNRT, corrected sinus node recovery time; EPS, electrophysiological study; ICM, implantable cardiac monitor; HV, His to ventricle; msec, milliseconds; VT, ventricular tachycardia.
Values are expressed as absolute numbers, No. (%), or median [interquartile range].
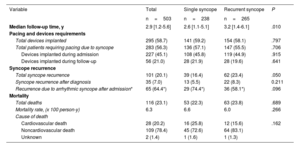
A total of 295 (58.7%) patients required device implantation at the end of follow-up (table 3), ( shows the type of device implanted). In most patients (283 [56.3%] patients), the indication was bradycardia related to the syncope. Two implantable cardiac defibrillators and 2 cardiac resynchronization defibrillators were implanted due to VT. Three patients with VT were treated with antiarrhythmic drugs only due to their comorbidities. Additionally, 5 pacemakers were implanted due to postsurgical AV block and 3 additional pacemakers because of chronotropic insufficiency.
Outcomes during follow-up
| Variable | Total | Single syncope | Recurrent syncope | P |
|---|---|---|---|---|
| n=503 | n=238 | n=265 | ||
| Median follow-up time, y | 2.9 [1.2-5.6] | 2.6 [1.1-5.1] | 3.2 [1.4-6.1] | .010 |
| Pacing and devices requirements | ||||
| Total devices implanted | 295 (58.7) | 141 (59.2) | 154 (58.1) | .797 |
| Total patients requiring pacing due to syncope | 283 (56.3) | 136 (57.1) | 147 (55.5) | .706 |
| Devices implanted during admission | 227 (45.1) | 108 (45.8) | 119 (44.9) | .915 |
| Devices implanted during follow-up | 56 (21.0) | 28 (21.9) | 28 (19.6) | .641 |
| Syncope recurrence | ||||
| Total syncope recurrence | 101 (20.1) | 39 (16.4) | 62 (23.4) | .050 |
| Syncope recurrence after diagnosis | 35 (7.0) | 13 (5.5) | 22 (8.3) | 0.211 |
| Recurrence due to arrhythmic syncope after admission* | 65 (64.4*) | 29 (74.4*) | 36 (58.1*) | .096 |
| Mortality | ||||
| Total deaths | 116 (23.1) | 53 (22.3) | 63 (23.8) | .689 |
| Mortality rate, (x 100 person-y) | 6.3 | 6.6 | 6.0 | .266 |
| Cause of death | ||||
| Cardiovascular death | 28 (20.2) | 16 (25.8) | 12 (15.6) | .162 |
| Noncardiovascular death | 109 (78.4) | 45 (72.6) | 64 (83.1) | |
| Unknown | 2 (1.4) | 1 (1.6) | 1 (1.3) |
Values are expressed as No. (%) or median [interquartile range].
Arrhythmic syncope was identified in 291 (57.9%) patients, mostly secondary to bradycardia, especially aAVB/sCD (figure 3 and ). summarizes the differences in the baseline characteristics between of patients with and without an arrhythmic syncope. shows the arrhythmic risk according to the type of cBBB.
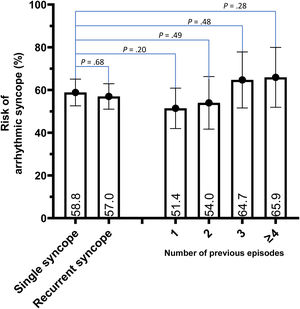
Arrhythmic risk was similar in patients with and without an SSE (58.8% [95%CI, 52.6%-65.1%]) vs 57.0% [95%CI, 51.0%-62.9%]), representing a risk ratio of 0.97 (95%CI 0.83-1.12) (figure 3). Furthermore, in the multivariate Cox model, after adjustment for possible confounding variables (including the type of cBBB), the presence of recurrent syncopal episodes was not associated with a higher risk of an arrhythmic syncope (HR, 1.06; 95%CI, 0.81-1.38; P=.674) ().
Although a linear trend between the number of previous syncopal episodes and the risk of arrhythmic syncope was observed in the RSG (MH test for linear trend: 3.9; P=.0487), no significant differences were found between the number of previous syncopes compared with an SSE (figure 3).
Follow-up: recurrences and prognosis (secondary outcomes)Patients were followed up for a median of 2.9 [IQR, 1.2-5.6] years. After hospital admission, 101 (20.1) patients had a recurrence of syncope (table 3). In most of them (66 patients), the recurrence occurred before the cause of syncope was established, and indeed it was used to reach the diagnosis in step 3. The recurrence was due to an arrhythmic cause in 74.4% of patients with SSG and in 58.1% of patients with RSG (P=.096). Importantly, once the etiological diagnosis was made and the appropriate treatment established, only 35 patients (7.0%) experienced another syncopal recurrence (13 [5.5%] patients in SSG and 22 [8.3%] in RSG, P=.211), most of them due to orthostatic or reflex mechanisms (). The recurrence incidence rate in the SSG group was 1.7 per 100 person-years and 2.2 per 100 person-years in RSG (IRR, 0.76; 95%CI, 0.35-1.58).
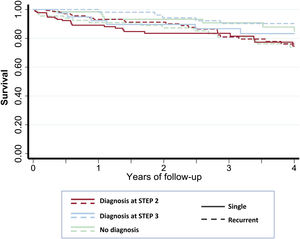
A total of 116 (23.1%) patients died during the follow-up, 78.4% of them due to noncardiovascular causes (table 3). The mortality rate in the SSG and RSG was 6.6 per 100 person-years and 6.0 per 100 person-years respectively (IRR, 1.12; 95%CI 0.76-1.64). Figure 4 shows Kaplan-Meier survival curves according to the time of diagnosis and groups.
DISCUSSIONIn this large observational cohort study, we compared the risk of arrhythmic syncope associated with a history of previous syncopal episodes in a population with unexplained syncope and cBBB. The main findings of the study are as follows: a) half of patients consulting for syncope had a previous history of at least 1 other episode; b) the risk of syncope of arrhythmic origin is high and is no different depending on the history of previous episodes; c) EPS and ICM offer a similar diagnostic yield in both groups; d) there are no clinically relevant differences between the 2 in terms of prognosis after a median of 3 years’ follow-up.
We found that over half of the patients reported 1 or more previous syncopal events, and up to one third of them had had at least 1 episode in the preceding 6 months. The risk of syncopal recurrences in the general population has been estimated at between 2 and 30% over a lifetime.20–23 However, the risk is likely much higher in those patients with conduction disturbances and with a higher mean age, as is the case in our study group. Similarly, in the B4 study3 and in the recently published Spritelly trial,24 which evaluated patients with syncope and bifascicular block, patients included reported a median of 2 previous syncopal episodes and in the ISSUE study25 the mean number of syncopal episodes during last 2 years was 3.
The main finding of our study is that a first syncopal episode and cBBB is associated with a high risk of arrhythmic origin, but this risk is similar to that of patients with recurrent episodes, even after adjustment for possible confounding variables, including the type of cBBB.
As far as we know, no previous studies have explicitly investigated this relationship. Some data on recurrence are available in previous studies that focus on other issues and with a more limited number of patients. In a previous study by our group, we found that the type of conduction disturbance pattern and PR interval was associated with the EPS result. However, the number of previous syncopal episodes was not correlated with the probability of having a positive EPS.11 In this respect, Azocar et al.26 investigated the diagnostic yield of a stepwise use of EPS and ICM in a cohort of 85 patients. They found that the risk of aAVB was higher in patients with prolonged PR or axis deviation, but no significant differences were found when comparing patients with single or recurrent episodes. Of note, these previous studies were not designed to investigate this relationship, and no statistical techniques were used to evaluate possible confounders or interactions. It is also worth mentioning that the EPS and ICM diagnostic yield was similar in both groups. The use of an ICM allowed for diagnosis during follow-up in half of patients with a negative EPS. This observation supports the findings of previous studies on the usefulness of early monitoring in patients with cBBB and negative EPS, even after the first syncopal episode.27
Prognosis was also found to be similar in both groups. As expected, in line with previous studies, patients without a diagnosis in step 2 had a higher risk of recurrence of syncope than those who were diagnosed in step 2, and the first recurrence of syncope led to the final diagnosis in step 3. After an etiological diagnosis, few patients had new recurrences (7.0%) and most of them were secondary to a nonarrhythmic mechanism. As the rate of recurrence is known to be reduced by treatment,1,5,28 this finding suggests that the diagnoses were specific and allowed for effective guidance of treatment in both groups. The appropriate treatment may also explain the fact that no differences in mortality were found, in contrast with previous studies.20,22,23
Overall, according to the results of this study, there is no clinical justification to forego a complete workup of syncope, or as an alternative to implant a permanent pacemaker in patients with an isolated syncopal episode and cBBB. Future clinical guidelines and recommendations should consider the findings of this study to improve patient care, adherence to recommendations, and avoid unnecessary delays in providing the right therapy.
LimitationsThis study has some limitations. It is an observational study carried out at a single high-volume center with a dedicated syncope clinic. To minimize the potential biases inherent to the study design, the patients were included consecutively. In addition, possible confounding factors were analyzed, including those related to a possible temporal bias due to the relatively long inclusion period. The reasons why the patients had not consulted in previous episodes were not analyzed. Patients were included in the study after step 1, and so this series refers not to the global etiology of syncope in this population, but rather on those patients lacking an evident initial diagnosis. Patients with isolated/single right cBBB were not excluded. Even though the arrhythmic risk in this subgroup of patients is significantly lower than those with other types of cBBB,11 arrhythmic risk is still significant (around 1 in 4) and similar in the 2 groups (), and therefore the overall conclusion of this study should not be affected. Furthermore, the type of cBBB was included as a variable in the multivariate analysis. In addition, the tilt-test was not used in the workup protocol due to its low specificity in this population.1 However, in selected patients, the tilt-test could have revealed an indication for pacing.1 Moreover, the study was not designed to assess predictors of pacemaker implantation in the 2 groups.
CONCLUSIONSPatients with cBBB and unexplained syncope are at high risk for an arrhythmic etiology, even following the first episode of syncope. Compared with patients with recurrent episodes of syncope, those with an isolated/a single episode have a similar arrhythmic risk, a similar incidence rate of recurrences after treatment, a similar prognosis, and a similar test diagnostic yield. To ensure that all patients receive the right therapy at the right time, future clinical guidelines should reinforce the need for similar management of patients with cBBB and unexplained syncope, regardless of prior history of syncope.
FUNDINGThis project was funded by ISCIII, CIBER and Fundació Marató TV3 and cofunded by the European Regional Development Fund (ERDF-FEDER).
AUTHORS’ CONTRIBUTIONSJ. Francisco-Pascual prepared the concept and led the study design, performed the statistical analyses, and designed, drafted and edited the manuscript; M. Maymi-Ballesteros, C. Badia-Molins and M. Bach-Oller helped to collect and review the data. N. Rivas-Gándara and B. Benito helped to prepare the concept and reviewed the data and results. All other authors helped to include patients in the study, collecting data and reviewing the manuscript. All authors agreed with the content of the final version of the manuscript.
- -
Arrhythmia, specifically paroxysmal advanced atrioventricular block, is the most common cause of unexplained syncope in patients with bundle branch block. However, up to nearly 40% of cases may be due to a nonarrhythmic cause.
- -
Clinical practice guidelines recommend either systematic study of the potential cause or empirical pacemaker implantation. However many patients are managed more conservatively, especially if the syncopal episode is the first.
- -
Half of the patients who consult for syncope and cBBB report at least 1 previous episode.
- -
Compared with patients with recurrent episodes of syncope, those with an isolated/single episode have a similar arrhythmic risk, a similar incidence rate of recurrences after the treatment, and a similar prognosis.
- -
According to the results of this study, there is no clinical justification to forego a complete workup of syncope, or as an alternative, to implant a permanent pacemaker in patients with an isolated/a SSE and cBBB.
The Vall d’Hebron Arrhythmia Unit receives fellowship grants from Boston Scientific and research grants from Abbott. J. Francisco-Pascual receives advisory and speaking honoraria from Abbott and Microport. N. Rivas-Gándara receives advisory and speaking honoraria from Abbott. B. Benito, J. Pérez-Rodón and A. Santos-Ortega receive speaking honoraria from Abbott. The other authors report no conflicts of interest.
The authors would like to thank the staff of the cardiology department and arrhythmia unit for their support in patient management, monitoring, and follow-up.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.009