One of the aims of secondary prevention is to achieve plaque stabilization. This study sought to investigate the clinical consequences and predictive factors of the change in the type of plaque (CTP) as assessed by serial intracoronary ultrasound in type II diabetic patients with known coronary artery disease.
Methods237 segments (45 patients) from the DIABETES I, II, and III trials were included. Intracoronary ultrasound from motorized pullbacks (0.5mm/s) after index procedure and at 9-month angiographic follow-up was performed in the same coronary segment. Nontreated mild lesions (angiographic stenosis<25%) with ≥0.5mm plaque thickening and ≥5mm of length assessed by intracoronary ultrasound were included. As different types of plaques may be encountered throughout a given coronary lesion, each study lesion was divided into 3 segments for serial quantitative and qualitative analyses. Statistical adjustment by multiple lesion segments per patient (generalized estimating equations method) was performed. A CTP was defined as any qualitative change in plaque type at follow-up. At 1-year follow-up, major adverse cardiac events – death, myocardial infarction and target vessel revascularization) – were recorded.
ResultsA CTP was observed in 48 lesions (20.2%) and occurred more frequently (52.1%) in mixed plaques. Independent predictors of CTP were glycated hemoglobin levels (odds ratio [OR] 1.2; 95% confidence interval [CI] 1.01-1.5; P=.04); glycoprotein IIb-IIIa inhibitors (OR 0.3; 95% CI 0.1-0.7; P=.004) and statin administration (OR 0.3; 95% CI 0.1-0.8; P=.02). At 1-year follow-up CTP was associated with an increase in major adverse cardiac events rate (CTP 20.8% vs non-CTP 13.8%, P=.008; hazard ratio=1.9, 95% CI 1.3-1.9, P=.01).
ConclusionsQualitative changes in mild stenosis documented by intracoronary ultrasound in type II diabetics are associated with suboptimal secondary prevention and may have clinical consequences.
Keywords
.
INTRODUCTIONIntracoronary ultrasound (ICUS) is able to characterize plaque composition based on its echogenicity.1 In fact, ICUS has been shown to predict histological characteristics of atherosclerotic plaques in 96% of patients studied at necropsy.2 Four basic types of components that correspond to different echogenic signal have been described: lipid deposit (hypoechoic), fibromuscular tissue (soft echoes), fibrous tissue (bright echoes), and calcium deposits (bright echoes with shadowing behind the lesion).3 Similar findings were observed from histologic samples obtained during directional coronary atherectomy showing that plaques with larger fractions of dense fibrous, elastic, or calcified tissue are predominantly echogenic, whereas echolucent soft plaques have a greater fraction of smooth muscle, thrombotic, or necrotic elements.4
Assessment of plaque morphology by ICUS, independently of the severity of the underlying stenosis, has led to a better understanding of the pathophysiology of coronary atherosclerotic disease and its clinical consequences in cross-sectional studies.5, 6 Thus, plaques with a larger percentage of lipid area and with a thin fibrous cap are more prone to rupture than fibrous plaques.7 In this regard, vulnerable plaques which can lead to acute coronary events are more often seen in type II diabetic patients than in nondiabetics.8 To achieve plaque stabilization by decreasing the amount of lipids within the plaque, statin treatment has been advocated for use in secondary prevention.9
To date, the clinical impact of change in the type of plaque (CTP) in type II diabetics, as assessed by serial ICUS analyses, has not been prospectively evaluated. Thus, we hypothesized that detection of dynamic plaque changes may identify patients at risk of subsequent coronary events. Therefore, we designed this ICUS study to assess the clinical consequences and the predictive factors of CTP in type II diabetic patients with known coronary artery disease.
METHODS Study PopulationType II diabetic patients enrolled in the DIABETES (DIABETes and sirolimus Eluting Stent) I, II, and III trials10, 11, 12 in whom ICUS evaluation was performed were included in this study. The flow chart of the study and inclusion and exclusion criteria have been previously described.13 Briefly, nontreated mild lesions (angiographic stenosis<25% by visual assessment) with a plaque thickening of ≥0.5mm and length of ≥5mm as assessed by ICUS were selected. Coronary segments eligible for serial analyses had to be located at least 10mm distal or proximal from the previously stented segment and thus not subject to balloon injury during the index procedure. We excluded from the analysis those lesions with artifacts related to ICUS, such as nonuniform rotational distortion, ring down, lesions with incomplete circumference, and ICUS pullback that did not include the lesion at follow-up, and lesions that were treated before 9 months of follow-up. In addition, patients with multiple balloon inflations during complex procedures were excluded when doubts remained concerning the exact location of the inflated balloon. Study protocols were approved by the medical ethics committees of the participating institutions and all patients gave written informed consent.
Intravascular Ultrasound Imaging and AnalysisQualitative and quantitative analyses from motorized pullbacks (0.5mm/s) after the index procedure and at 9-month angiographic follow-up were performed in the same coronary segment. In all cases, the ICUS system used was the ClearView™ console (CVIS, Sunnyvale, California) with the Atlantis-Pro 40MHz catheter™ (CVIS, Sunnyvale, California). Angiographically nonsignificant plaques not related to the treatment site were serially analyzed. An individual plaque was defined as a continuous atheroma with plaque plus media thickness >0.5mm and length of ≥5mm, and with no intervening branches. A disease-free segment of at least 5mm was required to differentiate plaques. Adjacent segments with no intervening significant side branches, meeting the normal criteria and >2.5mm in length, were used as references.
Further, as different types of plaques may be encountered throughout a given coronary lesion, each study lesion was divided into 3 segments for serial quantitative and qualitative analyses.14 This serial ICUS analysis was performed by an independent core lab (University of Florida Health Science Center at Shands Jacksonville, Florida), blinded to clinical and laboratory data. The analyses followed a previously described methodology.15 From the digitized images, lumen, plaque and external elastic membrane (EEM) areas were measured at intervals of 0.5mm in each coronary segment. Mean lumen, plaque and EEM areas from baseline to 9-month follow-up were calculated. The distance from plaque to the previous implanted stent and the distance from the plaque to any side branch were recorded to ensure analyses of the same coronary segment at follow-up. Quantitative three-dimensional ICUS analysis was performed using a dedicated ICUS analysis system (QIVA, Pie Medical Imaging, Maastricht, The Netherlands). This system enables semiautomated contouring of the lumen and vessel, as well as quantitative analysis of their dimensions in longitudinal and cross-sectional views. Locations of the minimal and maximal cross-sectional areas (lumen, plaque, and EEM) were automatically defined by the computer algorithm. Measurements were taken along the entire plaque and any related references. Volumes were determined from a summation of measured cross-sectional areas of the pullback region based on Simpson's rule. For calcified cross-sections, the contour of the EEM was interpolated from noncalcified slices. In addition, segments were qualitatively categorized by 2 experienced observers into 4 morphological categories: soft, fibrous, mixed, and calcified plaques. Intraobserver variability was assessed by analyzing a series of 40 segments at least 3 months apart. The proportion of agreement is >95% (Kappa=0.946 for baseline type of plaque and Kappa=0.965 for follow-up type of plaque); in cases of disagreement between the two observers the opinion of a third observer was required.
DefinitionsThe type of plaque was defined as follows16: soft tissue when at least 80% area was constituted by material showing less echo reflectivity than the adventitia, with an arc of calcium <10 degrees; fibrous plaque when echo reflectivity of at least 80% of the material was as bright as or brighter than the adventitia without acoustic shadowing; diffuse calcified plaque when it contained material brighter than adventitia, showing acoustic shadowing in >90 degrees; and mixed when plaque did not match the 80% criterion. A CTP was defined as any qualitative change in plaque type from the index procedure to 9-month follow-up. Serial vascular changes were categorized as CTP or no-CTP.
Quantitative serial vascular changes were categorized as vessel shrinkage or vessel enlargement. Vessel shrinkage was defined as the ratio of delta vessel area to delta atheroma area <0.13, 17
Major adverse cardiac events (MACE) were defined as cardiac death, myocardial infarction (MI), and target vessel revascularization (TVR) and were adjudicated at 12-month follow-up.
Statistical AnalysisStatistical analysis was performed with the SPSS 12.0 and SAS 9.1 versions. Quantitative data are presented as mean±standard deviation and qualitative as percentages. To take into account the intra-individual variability (repeated assessments), all comparisons (univariate and multivariate) were adjusted by a generalized estimating equations [GEE] model stratifying by patient, lesion, and segment.18 To evaluate the association between CTP and clinical, laboratory, and quantitative ICUS data, a multivariate logistic regression model was performed. Those variables with a probability value <0.1 on univariate analysis or clinically relevant were included in the analyses: glycated hemoglobin, low-density lipoprotein (LDL) and triglyceride levels, age, smoking, hypercholesterolemia, stable angina and diabetes, baseline mean vessel area, baseline mean plaque area, baseline type of plaque, and the use of statins, angiotensin converting enzyme (ACE) inhibitors, and glycoprotein (GP) IIb-IIIa inhibitors. A two-tailed value of P<.05 was considered statistically significant.
RESULTS Baseline Characteristics and Intracoronary Ultrasound ResultsSerial ICUS analyses of 237 matched atherosclerotic segments from 45 patients were analyzed at baseline and at 9-month follow-up. The baseline characteristics of the patients included have been previously described13 (Table 1).
Table 1. Baseline Characteristics (n=45).
| Age (years) | 67.4±9.1 |
| Female | 19 (22.2) |
| IDDM | 13 (28.9) |
| Hyperlipidemia | 29 (64.4) |
| Hypertension | 30 (66.7) |
| Smoke | 26 (57.8) |
| Previous MI | 19 (42.2) |
| Previous revascularization | 8 (17.8) |
| Stable angina | 11 (24.4) |
| Multivessel disease | 30 (66.7) |
| LVEF | 66.6±14.1 |
| Statins | 37 (82.2) |
| GPIIb-IIIa inhibitors | 26 (57.8) |
| Glycated hemoglobin | 7.2±1.4 |
IDDM, insulin dependent diabetics; GPIIb-IIIa, glycoprotein IIb-IIIa; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Data presented as mean±standard deviation or n (%).
Mean lesion length was 10.3mm. The type of plaque more frequently observed was mixed (39.2%), followed by calcified (31.3%), fibrous (16.5%), and soft (13.1%). A CTP occurred in 48 lesions (20.2%) (Figure 1). The type of plaque that more often changed during follow-up was mixed (52.1%), followed by fibrous (22.9%). A CTP occurred less often in soft and calcified plaques (12.5% in both types). Quantitative ICUS results are shown in Table 2. Delta values of vascular dimensions were comparable between the CTP group and the no-CTP group: vessel area (1.3±3.5 vs 1.1±3.6, P=.66), plaque area (0.6±2.1 vs 0.4±2.1, P=.86), and lumen area (0.7±2.5 vs 0.6±2.5, P=.7).
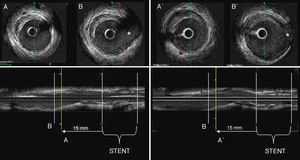
Figure 1. An example of an intracoronary ultrasound study of a patient included in the study that shows a change in the type of plaque from fibrous to soft. The left panel represents the baseline study that shows a fibrous plaque which is located proximal to the stent (15mm) and proximal to a landmark: B (asterisk: sidebranch). At the right panel the follow-up study showing a soft lesion (B) located at the same position. B’ represents the landmark at follow-up.
Table 2. Quantitative Intracoronary Ultrasound Measurement.
| Change in the type of plaque (n=48) | No change in the type of plaque (n=189) | P | |
| Baseline | |||
| Mean EEM area, mm2 | 19.3±3.5 | 20.5±5.1 | .07 |
| Mean plaque area mm2 | 9.3±3.1 | 10.3±3.4 | .07 |
| Mean lumen area, mm2 | 10±2.7 | 10.1±3.8 | .78 |
| Follow-up | |||
| Mean EEM area, mm2 | 20.6±4.4 | 21.5±5 | .15 |
| Mean plaque area, mm2 | 9.9±3.3 | 10.8±3.5 | .22 |
| Mean lumen area, mm2, | 10.7±3.4 | 10.8±3.9 | .91 |
| Vessel shrinkage, n (%) | 16 (33.3) | 72 (38.1) | .7 |
EEM: external elastic membrane.
Continuous data expressed as mean±standard deviation or n (%). All statistics were calculated with the generalized estimating equations method stratifying by patients, vessel and segment.
The clinical analysis of the CTP and no-CTP groups is shown in Table 3. The CTP group was significantly older and with a higher prevalence of insulin dependent diabetics, and lower prevalence of hyperlipidemia, smoking, and use of statin, ACE inhibitors and GPIIb-IIIa inhibitors. Lower LDL cholesterol levels and a trend toward higher glycated hemoglobin levels were observed in the CTP group at baseline. There was no relationship between quantitative vessel changes and CTP or between baseline plaque type and CTP. In multivariate analyses the factors independently associated with CTP were glycated hemoglobin levels (P=.04), the use of GPIIb-IIIa inhibitors (P=.004), and statin use (P=.02) (Table 4).
Table 3. Stratified Univariable Analysis Between the Lesions that Changed in the Type of Plaque and Those that Did not Change at Follow-up.
| Change in the type of plaque (n=48) | No change in the type of plaque (n=189) | P | |
| Age (years) | 69.4±7 | 66.5±9.7 | .024 |
| Male | 43 (75.4) | 146 (81.1) | .35 |
| IDDM | 18 (31.6) | 35 (18.5) | .028 |
| Hyperlipidemia | 23 (47.9) | 136 (72) | .002 |
| Hypertension | 33 (68.8) | 126 (66.7) | .78 |
| Smoke | 23 (47.9) | 136 (72) | .007 |
| Previous MI | 17 (35.4) | 79 (41.8) | .42 |
| Previous revascularization | 17 (35.4) | 79 (41.8) | .42 |
| Stable angina | 18 (37.5) | 45 (23.8) | .057 |
| Multivessel disease | 35 (72.9) | 133 (70.4) | .72 |
| LVEF (%) | 63.8±16.9 | 66±16.6 | .4 |
| Statin | 32 (66.7) | 172 (91) | <.001 |
| ACE inhibitors | 23 (47.9) | 121 (64) | .04 |
| GPIIb-IIIa inhibitors | 21 (43.8) | 111(58.7) | .06 |
| Total cholesterol, mg/dl | 175.9±52.8 | 166.1±29.5 | .15 |
| Triglycerides, mg/dl | 124.7±48.9 | 141.3±61.6 | .09 |
| HDL- cholesterol, mg/dl | 41.9±9.3 | 42.5.4±12.6 | .74 |
| LDL- cholesterol, mg/dl | 108.5±45 | 195.0±32.3 | .03 |
| Apolipoprotein A, mg/dl | 118.4±27.3 | 119.1±24.4 | .88 |
| Apolipoprotein B, mg/dl | 98.3±32.7 | 93.6±21.6 | .38 |
| Glycated Haemoglobin (%) | 7.8±1.2 | 7.4±1.4 | .059 |
| hs-CRP | 0.7±0.7 | 1.1±1.5 | .23 |
ACE, angiotensin converting enzyme; GPIIb-IIIa, glycoprotein IIb-IIIa; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein; IDDM, insulin dependent diabetic; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Data presented as mean±standard deviation or n (%). All statistics were calculated with the generalized estimating equations method stratifying by patients, vessel and segment.
Table 4. Stratified Multivariate Analyses for Predictors of Change in the Type of Plaque.
| OR | 95% CI | P | |
| Glycated hemoglobin | 1.25 | 1.01-1.56 | .04 |
| Triglycerides | 0.99 | 0.98-1 | .08 |
| Hypercholesterolemia | 0.55 | 0.25-1.19 | .12 |
| Smoking | 0.59 | 0.28-1.27 | .18 |
| Baseline mean vessel area | 0.93 | 0.86-1.01 | .08 |
| Statin | 0.35 | 0.14-0.88 | .02 |
| GPIIb-IIIa inhibitors | 0.34 | 0.16-0.72 | .004 |
CI, confidence interval; GPIIb-IIIa, glycoprotein IIb-IIIa; OR, odds ratio.
All statistics were calculated with the generalized estimating equations method, stratifying by patients, vessel and segment.
At 1-year clinical follow-up there was a significant increase in overall MACE in the CTP group, [13 (27.1%) vs 29 (15.3%); P<.001] mainly due to a significant increase in the need of TVR [10 (20.8%) vs 26 (13.8%)]; P=.008. In the CTP group, TVR was due to progression of atherosclerosis in other coronary segments in 6 cases and target lesion revascularization due to restenosis was needed in 4 cases. In the no-CTP group, TVR was due to progression of atherosclerosis in other coronary segments in 20 cases whereas 6 cases presented target lesion revascularization. Only in 1 case was the culprit artery in a myocardial infarction related to previous mild plaques analyzed and confirmed by ICUS. There was no cardiac death in either group. In addition, there was a trend toward higher frequency of MI in the CTP group [3 (6.3%) vs 3 (1.6%); P=.08].
The CTP was also an independent factor associated with MACE at 1 year (hazard ratio=1.9; 95% confidence interval 1.3-9.9; P=.01) adjusted by age, type of diabetes, and multivessel disease. The direction of plaque changes and clinical events at 1-year follow-up in the CTP group are depicted in Table 5.
Table 5. Direction of Plaque Changes and Clinical Events at 1 Year Follow-up
| Baseline type of plaque | Follow-up type of plaque | Number of events at 1 year in the CTP group (n=13) |
| Soft (n=6) | Mixed (n=6) | Events n=3 (n=2 TVR/n=1 TLR) |
| Mixed (25) | Soft (n=17) | Events n=3 (n=1 TVR/n=1 MI/n=1 TLR) |
| Fibrous (n=4) | - | |
| Calcified (n=4) | Events n=2 (n=1TVR/n=1TVR) | |
| Fibrous (n=11) | Mixed (n=10) | Events n=4 (n=1 TVR/n=1 TLR/ n=2 MI) |
| Calcified (n=1) | - | |
| Calcified (n=6) | Mixed (n=6) | Events n=1 TLR |
CTP, change in the type of plaque; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
Exclusive events.
The main findings of the present study are: a) 20% of the studied plaques experienced qualitative changes at 9-month follow-up; b) poor glycemic control was positively associated with this change whereas statins and GPIIb-IIIa inhibitors prevented its occurrence; and finally, c) CTP appeared to be related to clinical outcomes at 1 year.
It has been suggested that the type of plaque rather than the severity of the underlying stenosis plays a role in the propensity to vulnerability and therefore the development of an acute coronary syndrome.5, 6, 19, 20 Pathology studies showed lipid-rich plaques are more prone to rupture than are fibrotic plaques.21 ICUS studies described that plaques containing echolucent zones have a greater tendency towards instability. In addition, the type of plaque assessed by ICUS is associated with clinical events. Nakamura et al.22 showed that soft plaques are more prone to develop positive remodeling and acute coronary syndromes, and are a predictor of intra-stent restenosis.23
This is the first study that demonstrates that CTP in type II diabetic patients with known coronary artery disease has clinical implications. The change that occurs in one atherosclerotic plaque seems not to be an isolated phenomenon limited to one coronary segment but a “pancoronaritis” as suggested in recent ICUS and angioscopic studies.24, 25, 26 In this regard, patients with acute coronary syndromes studied by ICUS had plaque ruptures in locations other than the culprit lesion, even in different arteries. This suggests that systemic factors in addition to local factors probably influenced the entire coronary tree and led to a diffuse destabilization of coronary plaques. This is supported by previous studies showing a widespread neutrophils activation across the coronary tree regardless of the culprit lesion in patients with unstable angina.27 One may then hypothesize that CTP in mild lesions remote from a previously treated coronary segment may be the reflection of a process that involves the entire vessel.
Type II diabetic patients have more aggressive atherosclerotic disease and increased risk of thrombotic complications.28 Hyperglycemia plays a major role in the pathophysiology of vascular disease in diabetes that involves abnormalities in the endothelial and platelet functions. Hyperglycemia induces endothelial dysfunction via multiple mechanisms. It increases oxidative stress, leading to adventitial inflammation and vasa vasorum neovascularization, both of which are processes associated to plaque instability.29 In addition, poor glycemic control is an important factor in activating platelets that are key in atherogenesis, plaque progression, and thrombotic complications.30 In the present study higher levels of glycated hemoglobin led to CTP, emphasizing the important role of metabolic control to improve outcome in type II diabetic patients.
Statin administration has been associated with plaque regression and plaque stabilization based on the reduction in lipid content.9 In the present study statin use was a preventing factor for the appearance of CTP and therefore associated with better outcome. One possible explanation is that our patients were on chronic treatment with statins (61.8% simvastatin and 38.2% atorvastatin: 20-40mg) and LDL levels were stable over time: only 2 patients increased dosage due to inadequate lipid control. In addition, plaques with more lipid content such as soft plaques are infrequent in our population and may be another explanation of our findings. The anti-inflammatory properties of statins also may be important, by preventing any CTP.31
The administration of GPIIb-IIIa inhibitors has two main effects: the antithrombotic effect due to the inhibition of integrin GPIIb-IIIa receptor, which potently inhibits platelet aggregation and thrombus formation, and the anti-inflammatory effect due to inhibition of the interaction between platelets and leukocytes. In patients with unstable angina, MI, or percutaneous transluminal coronary angioplasty (PTCA), a systemic inflammatory response with an increased level of highly sensitive C-reactive protein, interleukin-6 and tumor necrosis factor has been described.32 Likewise, the degree of inflammatory cell activation after percutaneous intervention has been associated with an increased rate of events at both short- and long-term follow-up.33 On the other hand, the administration of GPIIb-IIIa inhibitors during a coronary percutaneous procedure was associated with suppression of periprocedure rise in markers of inflammation. Other properties of GPIIb-IIIa inhibitors consist of decreasing the levels of sCD40L and circulating leukocyte-platelet aggregates,34 both of which play an important role in the development and progression of atherosclerosis. In our study the GPIIb-IIIa inhibitors were administered per protocol independently of the complexity of PTCA procedure and prevented the appearance of CTP. In summary, we postulate that CTP reflects a change in the vessel and/or activation of the plaque that may be linked to events. Therefore, factors associated with plaque instability and plaque progression, such as higher levels of glycated hemoglobin, may explain the association with CTP.
Study LimitationsThis is an observational study in which the main limitation consists of the potential selection bias for two reasons: 1) only arteries suitable for ICUS examination were included and 2) patients with multiple balloon inflations during complex procedures were excluded when doubts remained concerning the exact location of the inflated balloon. In the present study only mild lesions were evaluated; therefore, the results derived from this study cannot be extrapolated to more severe lesions. The type of plaque was not associated with CTP. This finding may be related to small sample size. Similarly, only 6 soft plaques and 6 calcified plaques changed from baseline to follow-up and therefore, due to small sample size, we are not able to assess the implications of the different directions of plaque change. Probably the direction of CTP does not have the same clinical consequence and this constitutes a limitation of this study. Further studies with more patients or longer follow-up may be needed to determine the clinical consequences of a specific direction of change. However, we were able to demonstrate CTP in a rather high proportion of plaques, as well as a relationship between this change and clinical outcomes. These findings support the paradigm of the accelerated atherosclerosis process that occurs in the type II diabetic population.
Standard grey scale ICUS has significant limitations when it comes to tissue characterization. There is poor sensitivity, specificity, and reproducibility for plaque characterization based on visual assessment of the grey scale image, especially for lipid-rich plaques. For this reason the present study does not evaluate the change in plaque composition. This study tries to assess the clinical implications of CTP assessed by grey scale ICUS, with all the limitations that this technique has, including that ICUS image quality may be impeded by several artifacts and is dependent on the transducer position within the coronary artery. Moreover, noncoaxial eccentric catheter position results in geometric distortion and may affect the echogenicity of the vessel wall. Further, in vivo imaging is subject to changes during the heart cycle. Despite these limitations grey scale ICUS has been used to characterize plaque composition in large multicenter trials.9, 22, 23, 35
CONCLUSIONSQualitative changes in mild stenosis documented by ICUS in type II diabetics are associated with suboptimal secondary prevention and may have clinical consequences. The role of newer technologies such as Virtual Histology™ ICUS technology or the combination of optical coherence tomography and ICUS in this setting still need to be determined.
CONFLICTS OF INTERESTNone declared.
Received 2 September 2010
Accepted 21 January 2011
Corresponding author: Servicio de Cardiología Intervencionista, Hospital Clínico San Carlos, Plaza Cristo Rey s/n, 28040 Madrid, Spain. manelsabate1@telefonica.net