Keywords
INTRODUCTION
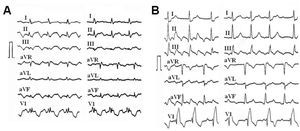
The term flutter describes an electrocardiographic pattern of atrial tachycardia >=240/min, with a uniform and regular continuous wave-form, contrasting with the P-waves separated by isoelectric lines which are characteristic of atrial tachycardia, with a rate <240/min (Figure 1). However, electrophysiological studies have revealed the mechanisms underlying ECG flutter and atrial tachycardia, thus making ECG-based classifications obsolete. In this work we attempt to present a current overview of atrial flutter and other mechanisms of macroreentrant tachycardia (MRT) in its clinical context and a description of the mapping and ablation methods that enable the invasive treatment of many of these circuits.
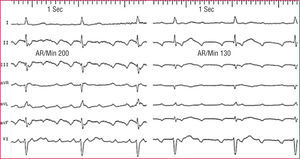
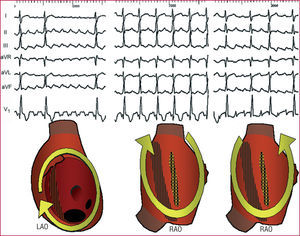
Figure 1. Patterns of typical atrial flutter (left) and focal atrial tachycardia (right) in a single patient. In both cases, apparently negative waves are recorded in the inferior leads, but in flutter there is a continuous wave, without a stable baseline, whereas in atrial tachycardia there are clearly defined isoelectric segments that separate the negative P waves in the lower wall. AR/min indicates atrial rate/min.
The term macroreentrant describes circular activation (reentrant) that revolves around a "large" obstacle, arbitrarily defined as being several centimeters in diameter.1 Typical atrial flutter, facilitated by the anatomical structure of the right atrium (RA)2,3 is the most frequent atrial MRT, but a growing incidence of MRT due to lesions, whether due to heart surgery with atriotomy or to left atrial (LA) ablation for the treatment of atrial fibrillation, complicates the treatment of patients receiving this treatment.
The relationships between flutter and atrial fibrillation are complex. Both arrhythmias frequently occur at various times in the same patient and epidemiological studies have demonstrated a tendency for patients who initially only present flutter to develop fibrillation after several years.4 Flutter and fibrillation could be expressions of a single arrhythmogenic substrate, which would make it necessary to fine-tune the concept of "curing" flutter, since catheter ablation would not have an effect on such a vaguely-defined substrate. Unfortunately, until recently, clinical trials grouped patients with both types of arrhythmias together and the signs identifying flutter remained hidden, being much less frequent than fibrillation. Thus, whereas we have direct information on the prognostic significance of fibrillation, or pharmacological treatment and anticoagulant therapy, little is known regarding flutter; the clinical recommendations are based on clinical observations and expert consensus rather than on evidence.5
TYPICAL ATRIAL FLUTTER
Mechanism of Typical Atrial Flutter
Typical atrial flutter,1 which is due to rotational activation around the RA (Figure 2), is the mechanism underlying 75%-80% of atrial MRT. The circuit is bounded in anteriorly by the tricuspid ring and posteriorly by a mixed anatomical and functional obstacle, formed by the venae cavae and crista terminalis.2,3 The activation wave-front goes down via the anterolateral RA and goes up via the septal RA (counterclockwise rotation in left anterior oblique view), necessarily passing between the inferior vena cava and the lower tricuspid ring, an area known as the cavotricuspid isthmus (CTI). Rotation in the high RA can occur in front of the superior vena cava or via a permeable part of the crista terminalis in other cases. Cycle length is typically 240-200 ms, with great stability (variations of <20 ms), but under pharmacological treatment or if there is delayed atrial conduction, the cycle length (CL) can reach 300 ms. This is a frequent situation in recurrent flutter following ablation.
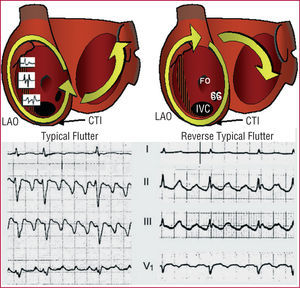
Figure 2. Mechanism and electrocardiographic pattern of typical atrial flutter (left) and reverse typical atrial flutter (right) in 2 different patients. The images show the atria in left anterior oblique view with the valvular rings expanded to show the position of the inferior vena cava (IVC), coronary sinus (SC), fossa ovalis (FO), and crista terminalis (CT). In typical atrial flutter, reentry around the tricuspid ring is anticlockwise (descending in the anterior RA and ascending in the septal RA) and in reverse typical atrial flutter it rotates clockwise. There is a functional block in the crista terminalis that produces double potentials. The cavotricuspid isthmus (CTI) is a necessary step in the circular activation wave-front. The left atrium undergoes passive activation from the circuit. For further details, see text. LAO indicates left anterior oblique.
Myocardial Anisotropy
The role of the crista terminalis is of great interest as a functional line of block because it highlights the importance of the myocardial structure in the conduction of the cardiac impulse. In the myocardial fiber bundles resistance is lower and the conduction velocity greater in the longitudinal direction than in the transversal direction across the fiber axis.6 This functional "asymmetry" is called anisotropy. Anisotropy is very marked in the crista terminalis because the low-resistance intercellular (gap) junctions have an end-to-end orientation, and conduction velocity can be 10 times greater in the longitudinal direction than in the transverse one.7 Transverse conduction via the crista terminalis is possible in many cases at low frequencies, but transverse block is the norm at the atrial flutter rate.8-10
Block in the crista terminalis causes double potential recordings, where each component shows activation on one side of the crista terminalis or the other11,12 (Figures 2 and 3). Anisotropy of the crista terminalis could be more accentuated in atrial flutter than in atrial fibrillation,9 which would suggest possible differences in the mechanism of arrhythmias with similar electrophysiological substrates. Anisotropy can be increased by antiarrhythmic agents,8 which would help explain why the patients treated with such agents for atrial fibrillation can develop flutter.13-15 Myocardial anisotropy can also be a determinant of the relative slowness of conduction in the CTI,16 where the direction of the myocardial fiber bundles overlaps and varies.17,18
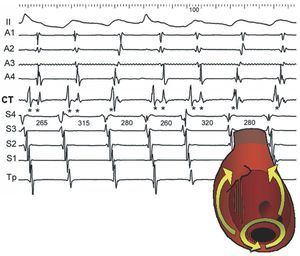
Figure 3. Double potentials as markers of conduction block in the crista terminalis (CT). This shows lead II and recordings of the anterior right atrium (RA) from top to bottom (A1-A4), medium-low CT, and low septal right atrium to high (S4-Tp). Typical atrial flutter, with block throughout the CT, alternates with lower loop reentry, with conduction in the traverse direction. Block in the CT produces double potentials (**) and descending activation of the anterior RA, whereas when there is conduction via the CT the local potential is single (*) and activation of the anterior RA is ascending. The septal RA is always activated from the bottom up. Values in ms.
The Right Atrium As an Ideal Substrate of Macroreentry
The combination of large valvular and venous orifices together with the functional obstacle in the crista terminalis makes the RA an ideal place for reentry and this is probably the means by which typical atrial flutter has such a characteristic electrocardiographic and functional image.19,20 The experimental model of flutter described in 194721 by Rosenblueth and García Ramos was based on an anatomical obstacle that joined the venae cavae with a crush injury in the posterior RA. This experimental lesion would replicate the role of the crista terminalis in human flutter. The irregular orientation of the myocardial fiber bundles in the CTI would induce a certain slowing of conduction in this location, which would help sustain the reentry circuit.
Reverse Typical Atrial Flutter
Anticlockwise rotation activation (going down via the anterolateral RA) occurs in 90% of typical flutter, but in 10% there is a reverse or clockwise rotation (going up the anterolateral RA and going down the septal RA)22 (Figure 2). The reason for this preference is unclear, but could also be related to myocardial anisotropy. It has been found that block occurs in the crista terminalis more easily in the medial-lateral direction than in the latero-medial,10 and in the CTI, as well as a greater facility for one-way clockwise block, which would facilitate the onset of reentry in the opposite direction (anticlockwise). In any case, in reverse typical atrial flutter the circuit is identical, the therapeutic approach the same and only the direction of activation and electrocardiographic pattern changes.
Variants of Typical Atrial Flutter
The crista terminalis can be permeable to conduction in the transverse direction at some points during flutter, such that the obstacle on the RA posterior wall would be of variable size.23 In most cases this only translates into activation patterns of the anterior RA which occur in the ascending part (from the point at which the crest is permeable). Double potentials are not recorded where the crest is permeable, only single ones, although they can be wide since conduction continues to be slow (Figure 3).
This has been called lower loop reentry, a circuit that only breaks around the IVC.24 These variants of typical atrial flutter are all treated similarly by ablation of the CTI, which continues to be a critical point in the circuit. Its relevance lies in the fact that ECG and electrogram patterns might be confusing, which should be taken into account during electrophysiological study (EP) in order to apply mapping and entrainment techniques at the right time.
Electrocardiogram of Typical Atrial Flutter
Typical atrial flutter has a characteristic pattern in leads II, III and/or aVF known as "common" and "sawtooth." The wave is complex with a slowly falling segment followed by a negative deflection that changes rapidly to positive to link with the following cycle (Figures 1, 2, and 4). The rate is usually between 250 and 320/min, but can be less in the presence of drugs or after CTI ablation. The pattern can be difficult to recognize if AV conduction is 2:1, but can be revealed by increasing the degree of block through massaging the carotid sinus or with intravenous verapamil, ATP or adenosine.
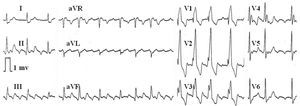
Figure 4. Variable atrioventricular conduction in a typical atrial flutter. The atrial wave pattern stays regular, at a constant rate, although the morphology changes at some points due to the overlapping T wave.
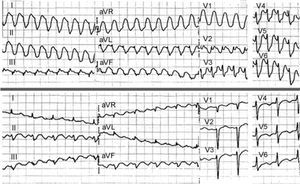
Reverse typical atrial flutter produces positive waves in lower leads, frequently with a sawtooth pattern, but what is most typical is a negative sawtooth wave, like a W, in V125 (Figures 2 and 5). The typical atrial flutter circuit can produce an atypical ECG in patients with organic heart disease, surgical atriotomy, or atrial ablation lesions.26 Flutter wave is partly due to LA activation, which would explain the lack of specificity of the ECG.27 In cases with atypical ECG, the electrophysiological study can only clarify the mechanism. In addition, a typical atrial flutter ECG in patients who have not undergone surgery or previous ablation predicts a typical circuit, dependent on the CTI with 90% probability (Figure 6).
Figure 5. Three macroreentrant tachycardias (MRT) of the right atrium (RA) in a single patient, undergoing surgery for interatrial septal defect. Left: reverse typical atrial flutter. The double negative waves ("W") in V1 are typical, whereas in II, III and aVF the continuous wave has a positive sawtooth appearance, but also has prominent negative deflections. Center: lesion-related macroreentrant tachycardia (MRT) with clockwise rotation in RAO view. 2:1 Atrioventricular conduction makes it difficult to distinguish the type of atrial wave, but it seems biphasic in II and aVF and positive in V1. Right: lesion-related MRT with anticlockwise rotation in RAO. The electrocardiogram presents a pattern indistinguishable from typical atrial flutter. RAO indicates right anterior oblique.
Figure 6. Combined view of the relationships between the mechanisms of atrial macroreentry and the electrocardiogram (ECG) patterns. The width of the triangle in each tachycardia mechanism indicates the relative frequency of this pattern with this mechanism. Atypical flutter denotes any atrial pattern with a continuous wave and rate (240/min), that does not meet the conditions of reverse typical or typical atrial flutter (see text). Atrial tachycardia denotes patterns with well-defined P waves between stable baselines with a rate of <240 beats/min. RA indicates right atrium; LA, left atrium; MRT, macroreentrant tachycardia; CTI, cavotricuspid isthmus.
Ventricular Response to Flutter
It is a classic paradox in atrial arrhythmias that a higher rate of atrial activation produces a greater degree of AV block and lower ventricular rate. AV conduction is frequently 2:1 during flutter, even while resting and under conduction-slowing agents; if the flutter rate is slower conduction can be up to 1:1 (Figure 7). However, ventricular rate can also be irregular due to complex block patterns in the AV node, which means that atrial fibrillation should not be systematically diagnosed when the ventricular response is irregular (Figure 4). The irregularity of the QRS complexes can make recognition of a regular atrial pattern difficult, but this does not present an insurmountable difficulty provided there is attentive, careful observation.
Figure 7. Upper: slow typical atrial flutter (200/min) with 1:1 AV conduction and wide QRS in a patient treated with flecainide for paroxysmal atrial fibrillation. Lower: some minutes later, there was a spontaneous change to 2:1 conduction with normalization of the QRS complex.
Pathogenesis of Typical Atrial Flutter
The background and diseases associated with flutter are similar to those of atrial fibrillation, including hypertension, coronary disease, valvulopathy, chronic obstructive pulmonary disease, myocardiopathy, and 15%-20% of apparently healthy hearts, but with 80% of individuals male.28-30 Dilatated RA is associated with flutter,31 whereas left ventricular dysfunction32 and dilatated LA are associated with the incidence of fibrillation after flutter ablation.33 Electrophysiological studies of flutter have demonstrated conduction delays similar to ones detected in fibrillation,34,35 which would tend to confirm the presence of a common electrophysiological substratum.
The clinical presentation of flutter is frequently associated with atrial fibrillation. In some cases, flutter initiates fibrillation and flutter ablation reduces the incidence of fibrillation.31 In other cases, the ectopic foci initiating fibrillation are the cause of flutter which disappears after the focii are ablated.36 Finally, flutter can appear in patients who initially only presented fibrillation, when being treated with antiarrhythmic agents.13,14
Clinical Characteristics
Flutter can be paroxysmal, with a similar clinical picture to that of other paroxysmal arrhythmias. When the patient has paroxysmal flutter and fibrillation, flutter tends to be more poorly tolerated due to the higher ventricular rate. During atrial flutter, AV conduction tends to be 2:1 with a stable ventricular rate of 130-150 beats/min, and responds with greater difficulty than fibrillation to digitalization or to beta-blockers and calcium antagonists. Occasionally, 1:1 AV conduction is found, with a ventricular rate of around 240-250 beats/min in the absence of preexcitation. When flutter is persistent, a high ventricular rate can cause dilated cardiomyopathy (tachycardia-induced cardiomyopathy), that is reversible.37 In other cases, especially in the elderly, AV conduction can be 3:1 or 4:1, with a well-tolerated or even slow ventricular rate.
Antiarrhythmic agents (amiodarone, flecainide, propafenone) can convert fibrillation into flutter, and also reduce flutter to ≤200/min. In this situation there may be 1:1 AV conduction with a wide QRS complex due to aberrant conduction, thus imitating a ventricular tachycardia13,14 (Figure 7).
It has been estimated that the incidence of systemic embolism during flutter is around one-third that of fibrillation.38 There are no studies that make it possible to assess the risk/benefit ratio of the various anticoagulation strategies in patients who present flutter only. When flutter is associated with fibrillation, risk is determined by the fibrillation.39
Treatment of Typical Atrial Flutter
Indications for treatment are marked by poor tolerance, the poor or adverse response to antiarrhythmic agents and the possibility of eliminating the circuit via catheter ablation. Studies comparing rhythm maintenance in relation to controlling the atrial fibrillation rate40-42 are not applicable to flutter, because there is poor clinical tolerance to the latter and due to the efficacy of ablation.
Cardioversion and Antiarrhythmic Drugs
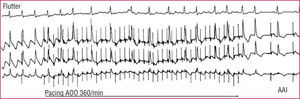
Electro-cardioversion of flutter has a success rate close to 100%, but flutter can also be interrupted in 85%-90% of cases with rapid RA pacing, using a temporary or permanent pacemaker43 (Figure 8). The availability of these 2 techniques makes it easy to resolve the problem of a very badly tolerated flutter with minimum risk.
Figure 8. Cardioversion of an atrial flutter by rapid pacing in a patient carrying an atrial demand inhibited pacemaker (AAI). Rapid pacing (spikes) totally changes the pattern of typical atrial flutter on the ECG and, when interrupting pacing, the rhythm is paced on demand by the pacemaker itself.
Pharmacological cardioversion of flutter with class Ic antiarrhythmic agents (flecainide, propafenone) is not indicated because, in contrast to atrial fibrillation, efficacy is reduced (nearly 40%) and, in addition, slowing the flutter can lead to 1:1 AV conduction (Figure 7).13,14,44-46 Intravenous administration of class III agents (dofetilide, ibutilide) would be effective in interrupting flutter. These primarily prolong the duration of the action potential without stopping myocardial conduction but, on the other hand, they incur the risk of causing polymorphic ventricular tachycardias (torsade de pointes).44 Such class III drugs are not available in Spain.
The incidence of recurrence of flutter after cardioversion could be lower than in fibrillation47,48 which means that, in the event that the arrhythmia is well-tolerated, a conservative strategy can be followed of clinical observation after the first episode has been cardioverted. On the other hand, if recurrence occurs or if the first episode is poorly tolerated, treatment should be via catheter ablation, which prevents recurrence in more than 90% of cases in the long term.30
When flutter is a result of treating fibrillation with antiarrhythmic agents, it is common to regard the appearance of flutter as a partial success and many of such patients stabilize in sinus rhythm. The antiarrhythmic agent is maintained after ablation of the flutter circuit and is known as hybrid therapy.49,50
Electrophysiological Study, Mapping, and Catheter Ablation
Flutter ablation is based on a well-established mechanical and anatomical foundation, that makes the CTI a necessary step in the circuit.51,52 The aim is bidirectional block of the CTI53,54 via radiofrequency or cryoablation. The clear definition of the anatomical target enables a procedure guided by the use of a reference multipolar catheter covering the anterior and septal RA to record the activation sequence, and another steerable catheter for mapping, pacing and ablation of the CTI.55,56 When ECG is typical and there is no background of atriotomy, CTI ablation can be done in sinus rhythm. However, if there has been previous surgery, when several circuits are possible, or when ECG is untypical or presents several patterns, it is essential to confirm the involvement of the RA and CTI in the circuit by mapping and pacing techniques during spontaneous or induced flutter.
Transient Entrainment of Flutter
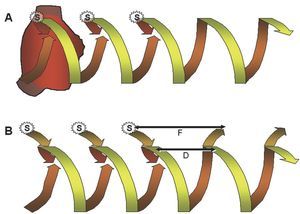
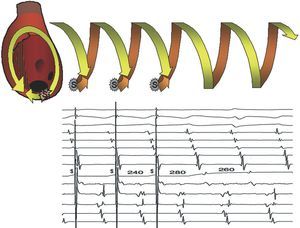
Transitory entrainment was described in patients with flutter in the period following open heart surgery,57 and later confirmed in other reentrant tachycardias. Pacing the high RA at a frequency higher than that of flutter accelerated the atrial rate, but kept the waves negative in the lower leads; when interrupting pacing, the baseline pattern and rate were reestablished. If pacing changed the polarity of the wave, flutter was interrupted and gave way to sinus rhythm. This acceleration of the circuit via pacing, while maintaining its configuration, is the essence of entrainment. In fact, it is the circuit that entrains activation, in the same way that rails keep a train on track, and this entrainment keeps the paced activation wave-front inside the circuit. Each pacing activates the circuit when penetrating it in the same direction as baseline activation and, at the same time, it pauses when penetrating it in the opposite57-59 direction (Figure 9).
Figure 9. Schematic representation of the entrainment mechanism of an MRT. Circular reentrant activation developing into helical form (spring or corkscrew) is shown with a time dimension. Upper (A): entrainment of typical atrial flutter by pacing (S) in the high RA. To the right, 2 spontaneous MRT cycles are shown, ascending in the septum and descending in the anterior RA. Each pacing initiates an activation wave-front in the anterior RA, the same as flutter, but earlier, and at the same time initiates another wave-front toward the septal RA that collides with the ascending activation wave-front of the flutter circuit. The last paced wave-front reinitiates the flutter circuit at the rate prior to pacing and the first return cycle is equal to the flutter cycle, due to being paced in the same circuit. Lower (B): when entrainment pacing is applied some distance from the circuit, the first unpaced cycle is equal to the baseline tachycardia within the circuit (D), but the conduction times to and from the circuit are added to this interval at the pacing point, which produces a longer cycle (F). In A and B there is a "fusion" between the circuit activation wave-front that remains equal to baseline and the one changed by the paced wave-front.
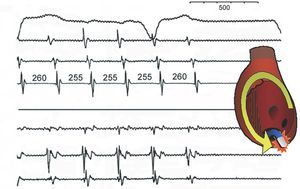
Entrainment has many interesting aspects but one is essential in practice: the duration of the return cycle (the first unpaced one) measured at the pacing point (Figure 9). If it is paced in the same circuit, the return cycle is similar (difference ≤20 ms) to baseline flutter. However, if the paced point is outside the circuit, the conduction times to and from the circuit are added to the return cycle of the tachycardia, which means that the return cycle at the pacing point is longer. Based on this principle, entraining flutter from the CTI confirms or rules out whether it is typical flutter (Figure 10).
Figure 10. Hidden entrainment of typical atrial flutter pacing the cavotricuspid isthmus (CTI). From top to bottom the diagram shows leads I, II, and V1 and recordings of the anterior RA, from above (Ta) to below (A4), then from the CTI and low septal RA (S4) to the roof (Tp). The upper panel schematically shows the activation sequence, descending in the anterior RA and ascending in the septal RA. The first three cycles are entrained (S), and from the CTI, and the cycle length (LC) of the entire circuit has been shortened to 240 ms; however, the anterior RA is still activated from top to bottom and there is no change in the ECG (hidden entrainment, without fusion). When interrupting pacing, flutter returns to the original wave-length (280 ms) and the return cycle in the CTI is equal to the flutter wave-length, which demonstrates that it is within the circuit.
Catheter Ablation
The ablation target is the CTI, being the narrowest part of the circuit, well-delimited anatomically, easily accessible and distant from the AV node60 (Figure 11). Ablation attempts to produce a complete, bidirectional and persistent CTI block, which normally requires several radiofrequency or cryoablation applications, between the tricuspid edge of the isthmus and the IVC. Some authors have suggested selecting the ablation points supported by entrainment,61,62 but this practice has been abandoned in favor of anatomical reference supported by the sequence of CTI electrograms. If ablation is done during flutter, the activation is interrupted abruptly when the CTI is blocked, thus confirming its role in the circuit. However, block that interrupts flutter can be transitory and it is usually necessary to continue ablation, while RA and CTI activation is verified by pacing one side and the other of the latter.53,54
Figure 11. Interruption of a typical atrial flutter during the application of radiofrequency (RF) in the cavotricuspid isthmus (CTI). Lead II and electrograms of the high anterior RA (HA), medial (MA), and low (LA), CTI and low septal RA (LS), medial (MS) and high (HS). The ascending activation in the septal RA follows the descending one in the anterior RA until the CTI is blocked, which stops the activation wave-front. Values in ms.
This maneuver is indispensable in confirming CTI block and minimizing recurrence (5%-10%). Once block is achieved it is necessary to verify its persistence over 20-30 min, as it can still recur.63,64
Ablation can be technically difficult when the myocardium of the CTI is thick and the anatomy irregular, thus hindering catheter stability.17,18 The current trend is to apply radiofrequency at up to 60-70 W with long electrode catheters (8-10 mm), or with saline-cooled electrode catheters that produce deep lesions at lower energies, thus obtaining bidirectional block in 85%-90% of cases.65,66 Radiofrequency application can be painful and requires sedation, whereas cryoablation is painless.67
Complications are rare. Apart from an incidence of vascular pr oblems in the femoral veins, <1% AV block has been described when the ablation is done on the septal RA, instead of on the CTI.68 Applications with an irrigated-tip ablation catheter may occasionally cause atrial perforation and cardiac tamponade.69
Following ablation the administration of antiarrhythmic agents is stopped, except in patients who developed flutter during pharmacologically treated fibrillation, in which case this must be continued permanently.49,50
Anticoagulation Therapy in Patients With Atrial Flutter
Data is extremely scarce on the incidence of systemic embolism in patients with flutter, and even less on the risk/benefit ratio of anticoagulation therapy.70-72 In general, the same guidelines have been adopted regarding anticoagulation therapy as in patients with fibrillation; however, in cases with a high risk of hemorrhage these standards could be made more flexible. Before cardioversion, either by direct current or pacing, the patient undergoes anticoagulation therapy for 3-4 weeks or thrombi in the left atrium (LA) are ruled out via transesophageal echography. Anticoagulation therapy is maintained for 4 weeks after cardioversion. In this sense, the interruption of persistent flutter by ablation is considered to be the same as cardioversion.71
Long-Term Prognosis
Once CTI block is obtained, flutter recurs in 5%-10% of cases, usually within 3 months, and reablation is, in general, technically easier.51,53,54,66-68 Some patients develop other atrial tachycardias or need a pacemaker due to concomitant sinus node dysfunction.29,73 Ablation can make the previously ineffective antiarrhythmic agents effective in the prevention of recurrent flutter, which, in selected cases, provides another alternative therapeutic option.
Although in some cases flutter ablation eliminates concomitant episodes of atrial fibrillation, a 25%-30% incidence of fibrillation is the main factor conditioning prognosis and quality of life of these patients.29,30,73-76 This incidence is greater in patients with fibrillation before ablation or if there is left ventricular dysfunction or LA dilatation. However, flutter ablation can still offer an interesting contribution to treatment in these cases, since flutter episodes tend to be more poorly tolerated and, when flutter occurs during pharmacologically treated fibrillation, flutter ablation enables controlling both arrhythmias in up to two-thirds of cases.49,50,76
Ablation can cure flutter, but clinical assistance should not stop here. A detailed history can reveal the presence of chronic bronchopneumopathy, sleep apnea, hypertension, obesity or other processes whose treatment could help stop the underlying arrhythmogenic process.
ATYPICAL ATRIAL FLUTTER AND ATYPICAL MACROREENTRANT TACHYCARDIA
Based on the definition of the typical atrial flutter circuit, other types of MRT have been found in both atriums, where the activation wave-front revolves around a great variety of organic and/or functional obstacles. Although the ECG is frequently classifiable as "atrial tachycardia," it is common to use the term flutter to designate these atypical reentrant arrhythmias (atypical flutter, left flutter) (Figure 6). Macroreentrant tachycardias due to atriotomy in the RA are well-known77 and, more recently, those caused by catheter ablation for atrial fibrillation.78
Right Atrial Macroreentrant Tachycardia
Atypical MRT is associated with structural heart disease, especially in those with a background of heart surgery. They are not dependent on the CTI and have as a central obstacle an surgical atriotomy lesion or low voltage areas that are considered lesion-like, usually linked to normal anatomical obstacles.
Lesion-Related Atypical Macroreentrant Tachycardia
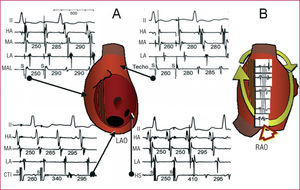
The simplest is based on a surgical lesion in the lateral RA, which forms a fixed obstacle on its own or is linked to the SVC by an area of functional block.79 The circuit has its rotation point (isthmus) at the lower margin of the lesion, near the IVC, or between two lesion areas of the lateral RA.80-82 Lesion-related atypical macroreentrant tachycardia (AMRT) almost always coexists with typical atrial flutter, and is frequently detected after the ablation of the latter, as a change in the activation sequence and the ECG takes place. Entrainment from the CTI and septal RA shows return cycles longer than that of tachycardia (Figure 12), whereas the return cycles through lateral RA pacing are the same as those of the tachycardia. Mapping the lateral wall shows a line of double potential electrograms from top to bottom, closed at its lower extreme by a fractionated electrogram, demonstrating slow conduction at the rotation point72,83,84 (Figures 12 and 13). The pressure of the mapping catheter at this point can stop the tachycardia, demonstrating its importance regarding the circuit; applying radiofrequency definitively interrupts the circuit. Reentry circuits have occasionally been described around an interatrial septal defect closure patch.85
Figure 12. Electrophysiological study of a macroreentrant tachycardia (MRT) of a lesion of the right atrium (RA) after CTI ablation due to typical atrial flutter. A: 4 panels with lead II and endocardial recordings of high anterior RA (HA), medial (MA), low (LA), and an exploratory electrode catheter used to entrain from various points. Activation of the anterior RA is descending. The return cycle is 340 ms in the CTI and 410 ms in the LS, longer than the baseline (280-295) cycle length (CL), because they lie outside the circuit. The return cycle is equal to the baseline in the medial anterolateral (MAL) RA and RA roof, which form part of the circuit. B: the MR circuit in right anterior oblique view. A line of double potentials is recorded in the center that converge in their lower part (arrow), where the tachycardia was interrupted by applying radiofrequency. Values in ms.
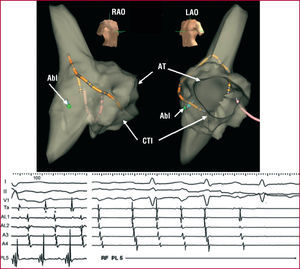
Figure 13. Mapping and ablation of typical atrial flutter and macroreentrant tachycardia (MRT) of a lesion in the right artery (RA) using the Navx system (r). Upper: virtual reconstruction of the RA anatomy in right anterior oblique (RAO) and left (LAO) views. Lower: recording of leads I, II, and V1 and endocardial high RA (Ta), high and medial anterolateral RA (AL1, AL2), and anterior RA. The positions of the stable mapping catheter in the RA and the spatial reference catheter in the coronary sinus can be identified. The diagnostic catheter (Abl) is in the posterolateral RA (PL5), where a wide fractionated potential was recorded. Note the descending activation of the anterolateral RA and the presence of double potentials in the high lateral RA (**) recordings, marking an area of block.
The lesion-related MRT ECG can be very similar to that of typical atrial flutter or completely different (Figures 5, 6, and 14) which means that, when there is a background of surgical atriotomy, interpreting the ECG is difficult and an electrophysiological study is essential to specify the diagnosis. The pattern is normally one of "atrial tachycardia" (Figure 14), with a frequency <240 beats/min and well-defined isoelectric lines. It is important to review the recorded ECG for various patterns that indicate the possible coexistence of more than one circuit, in order to plan the ablation.
Figure 14. Electrocardiogram of typical atrial flutter and lesion-related macroreentrant tachycardia (MRT) of the RA in 2 patients. A: male, 42 years, with Ebstein anomaly, operated on for interatrial septal defect closure. B: male, 40 years, operated on for interventricular septal defect closure. Typical atrial flutter is easily recognizable in the left panel in both cases. Lesion-related MRT inscribes isoelectric lines between P waves, especially in B, with a slower rate.
The treatment of lesion-related MRT is ablation of the lower isthmus, close to the IVC. The proximity of the phrenic nerve should be noted to prevent damaging it.85 Mapping such MRT can be facilitated by the use of virtual reconstructions of the atrial anatomy, which help guide the operator toward the critical points (Figure 13), although these techniques are not essential.
The prognosis of lesion-related AMRT after ablation is excellent. In our experience with 10 cases, there has been no recurrence of AMRT in the last 10 years, although there has been some recurrence of typical atrial flutter. Typical atrial flutter is the most frequent arrhythmia in patients who have undergone surgical atriotomy86 and CTI ablation, and is indicated in these cases, although typical atrial flutter has not been documented previously.
Atypical Macroreentrant Tachycardia Without Atriotomy
Macroreentrant tachycardia of the RA non-dependent on the CTI have been described, where the activation wave-front revolves at the SVC, also frequently including an area or line of functional block in the lateral RA.87-90 The lower rotation point of the circuit is usually in the lateral face of the atrium, near the IVC. An electrophysiological study would provide data similar to those of AMRT after atriotomy, and the ablation treatment would be similar. Reentry circuits contained within the SVC have been described.89 The long-term prognosis of these cases is not known, due to their limited frequency, and would be determined by the underlying disease.
Atypical Macroreentrant Tachycardia After Complex Congenital Heart Disease Surgery
The most challenging arrhythmogenic contexts are the Mustard, Senning, and Fontan procedures. In the Mustard and Senning procedures, not only are there complex incisions that can form the basis of a reentry circuit, but access to the circuit can be very difficult due to the presence of synthetic material or pericardial patches sutured to create the baffles.84,91 Even in these situations, the CTI-dependent flutter circuit is the cause of the tachycardia, but access to the CTI has to be done using the transaortic approach, or by crossing through the patches using a transeptal puncture technique, which involves a serious technical challenge.
After the Fontan procedure, large atrial dilatation with areas of myocardial fibrosis add to the surgical lesions, making delimitation of the specific circuits enormously complex.89,93 In such cases, ablation has been suggested during sinus rhythm, aimed at joining some fibrotic areas to others to stop the activation wave-front from rotating. Long-term prognosis is uncertain due to preexisting atrial damage and that added by the ablation procedure.
Focal mechanisms of tachycardia can be added to MRT, which makes evaluating these patients even more complex.92
Left Atrial Macroreentrant Tachycardia
This is usually associated with organic heart disease and/or serious alterations of the atrial myocardium.94 Left atrial MRT is usually based on wide electrically silent areas, considered lesion-like, although anatomical correlation is lacking.95,96
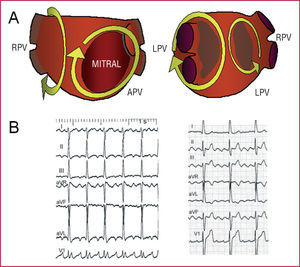
After mitral valve surgery, the surgical lesion may become the center of rotation.97 The left atrial MRT circuits can be very varied, without any predominating in particular. The activation wave-front can revolve around the mitral ring, pulmonary veins, or electrically silent areas (Figure 15). The ECG pattern is very variable, from an atypical flutter to a tachycardia at <200 beats/min (Figures 6 and 15). Voltage in the inferior leads tends to be low, whereas in the right precordial leads wide deflections are recorded.98 Wave-front activation in the RA is not usually circular, but could look similar in patients with functional or post-ablation CTI block, and ECG can look like typical atrial flutter in these patients. Entrainment from the RA will show long return cycles, although these can be short in the coronary sinus. Transeptal catheterization and electroanatomic mapping is required to define the circuits, with virtual reconstruction of the atrial anatomy in order to locate the critical isthmuses.
Figure 15. A: diagrams of different circuits described in macroreentrant tachycardia (MRT) of the left atrium (LA). On the left, anteroposterior view (APV); on the right, left posterolateral view (LPV). RPV indicates right pulmonary veins; LPV, left pulmonary veins. The irregular shaded areas are low voltage areas, attributed to myocardial fibrosis. B: electrocardiogram of 2 patients with MRT of the LA: one with a pattern indicative of atrial tachycardia (left) and one with a pattern of atypical atrial flutter (right).
The prognosis of left atrial MRT is uncertain. Wide series with long follow-up are not available and thus we do not know the risk-benefit ratio of ablating these circuits. At least 25% of patients can develop fibrillation during short-term follow-up96 and in the remaining patients the mechanical functioning of the LA is uncertain. Left atrial MRT ablation should be reserved for highly selected indications where the patient's quality of life is critically affected by the tachycardia which cannot be controlled with drugs. Atrioventricular node ablation followed by pacemaker implantation would be an alternative treatment in these cases.
The incidence of left atrial MRT has recently been recognized after circumferential pulmonary vein ablation in patients with atrial fibrillation,99,100 as a consequence of the non-transmural lesions created.101 The long-term prognosis of these arrhythmias is unknown, and remains one of the pending issues waiting resolution to know the long-term effect of these aggressive procedures in atrial fibrillation treatment.102
TYPE II FLUTTER
The term "type II flutter" was coined by Wells et al103 to designate a type of very fast, regular flutter (>340 beats/min), that could not be interrupted by pacing. Subsequently, this term has been used to designate any type of atypic al atrial flutter, although this name is inappropriate.1 Acceleration and change of activation of a flutter after overdrive pacing are frequently observed in the electrophysiology laboratory, that could be equivalent to the observations of Wells. There is insufficient data to assess the clinical significance of type II flutter.
Atrial Fibrillo-Flutter
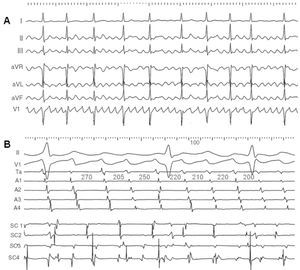
Some ECG recordings show wave-like atrial activity that at first glance suggests atypical atrial flutter, but following a more thorough analysis presents irregularities in frequency and morphology (Figure 16). Given the possibility of doubt, the term "fibrillo-flutter" has been applied to these cases. Endocardial mapping shows irregular changing activation patterns that indicate the absence of a dominant stable circuit. Fibrillo-flutter would be an arrhythmia more related to fibrillation than to flutter and the probability of being able to control it by ablation of a specific isthmus would appear slim.
Figure 16. Fibrillo-flutter. A: the electrocardiogram shows well-defined atrial waves that, at first glance, seem regular, but actually vary slightly regarding rate and morphology. B: the endocardial recordings show a strong variation in cycles and the activation sequence in the anterior RA (Ta to A4, from higher to lower) and a fractionated changing electrogram in the coronary sinus (SC1-SC4, in centimeters, proximal to distal, from the ostium). Values in ms.
Dr Ana Paula Magalhaes received a Fundación Miguel Servet grant from Medtronic Ibérica SA.
Correspondence: Dr. F. García Cosío.
Servicio de Cardiología. Hospital Universitario de Getafe.
Carretera de Toledo, km. 12,5. 28905 Getafe. Madrid. España.