Little is known about the characteristics of persons with familial hypercholesterolemia (FH) younger than 18 years, the lipid-lowering therapy used in these patients, and the lipid goals reached in real life. Our aim was to evaluate the achievement of low-density lipoprotein cholesterol (LDL-C) treatment goals in FH patients younger than 18 years enrolled in a large national registry.
MethodsWe analyzed patients younger than 18 years enrolled in a large ongoing registry of molecularly-defined patients with FH in Spain. The attainment of guideline-recommended plasma LDL-C goals at entry and follow-up was analyzed in relation to the use of lipid-lowering therapy.
ResultsWe enrolled 392 individuals younger than 18 years. Of these, 217 were molecularly-diagnosed FH patients and had a complete follow-up. The median follow-up time was 4.69 years (interquartile range, 2.48-6.38 years), 68.2% of FH patients were on statins, and 41.5% patients had LDL-C < 130mg/dL. Statin use was the only predictor of LDL-C goal attainment.
ConclusionsThis study shows that a high proportion of FH patients younger than 18 years have high LDL-C levels and fail to achieve recommended LDL-C targets. Statin use was the only independent predictor of LDL-C goal achievement. No safety concerns were detected during follow-up. These results indicate that many FH patients are not adequately controlled and that there is still room for treatment improvement.
Keywords
Heterozygous familial hypercholesterolemia (FH) is a common genetic disorder associated with premature atherosclerotic cardiovascular disease (ASCVD). Children with untreated FH are at increased risk of premature ASCVD after 20 years of age.1 The severe elevation of low-density lipoprotein cholesterol (LDL-C) levels begins in the fetus and leads to sustained exposure of the arterial wall to LDL-C, which accelerates cholesterol deposition and vascular inflammation and predisposes the early initiation of atherosclerosis, particularly in the coronary arteries and aorta.
Statins and other lipid-lowering therapies (LLTs) effectively lower LDL-C, are safe in children and adolescents, and restore endothelial function at an early age.2–4 Recently, universal screening of children from 2 years of age and before 8 years of age has been proposed5,6 to detect individuals requiring treatment. However, this approach is based on theoretical considerations and has not been proven in real life.
Nevertheless, little is known about the characteristics of FH patients younger than 18 years, the LLT used in these patients, and the lipid goals reached in real life. The information deficit is even greater for follow-up data. National registries can be used to provide this crucial information, which is necessary to improve models of care for FH, therapeutic protocols, and health policy.7,8 The SpAnish Familial HypErcHolEsterolaemiA CohoRt STudy (SAFEHEART) (NCT02693548) was designed to improve insight into the prognostic factors and mechanisms influencing the development of ASCVD and mortality in a FH population.
Our objective was to analyze patient characteristics and assess LLT and lipid goals at inclusion and during follow-up in FH patients younger than 18 years enrolled in SAFEHEART and to determine the factors predicting the likelihood of the attainment of these goals.
METHODSStudy Design and PopulationSAFEHEART is an open, multicenter, nationwide, long-term prospective cohort study in a molecularly-defined FH population in Spain. Recruitment of participants from FH families began in 2004 and is still ongoing. Inclusion criteria were index cases with a genetic diagnosis of FH and their relatives older than 15 years with a genetic diagnosis of FH, as well as their relatives without a genetic diagnosis of FH (control group). Nonetheless, participants younger than 15 years were also enrolled, if requested by their parents. This study was approved by the local ethics committees. All eligible individuals and/or at least 1 of their parents or legal guardians provided written informed consent. A coordinating center based in Madrid, Spain, was responsible for managing participant follow-up. Patients and/or their parents were contacted annually using a standardized telephone call to record relevant changes in lifestyle habits and medications and any cardiovascular events or other medical problems. Participating physicians who were enrolling patients and families in this registry received training, with best practice guidelines reinforced at annual meetings attended by physicians expert in the field; in addition, an electronically-based program and telephone advice were used and a web-based training program was deployed to further support management when required. Treatment decisions were exclusively made by each patient's physician.
Clinical and Laboratory MeasurementsDemographic and clinical characteristics were recorded as described elsewhere.9 Venous blood samples were taken after 12hours of fasting. Serum, plasma, and DNA samples were aliquoted and preserved at –80°C. Serum total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C) levels were measured in a central laboratory using enzymatic methods. Serum LDL-C concentration was calculated using the Friedewald formula. DNA was isolated from whole blood using standard methods and FH was genetically diagnosed using a DNA microarray.10 The LDL-C goals were defined according to recent recommendations and objectives. Low-density lipoprotein cholesterol < 130mg/dL was the primary goal.11 An alternative goal for patients younger than 14 years consisted of LDL-C < 160mg/dL in the absence of any other cardiovascular risk factors (smoking, HDL-C < 40mg/dL, lipoprotein (a) > 50mg/dL, or LDL-C > 250mg/dL) or premature cardiovascular disease in the progenitors or grandparents.6 Premature familiar ASCVD was defined as the occurrence of a first event before 55 years of age in men and before 65 years of age in women.
Lipid-lowering Therapy ClassificationMaximum statin dose was defined as atorvastatin 40 to 80mg/d or rosuvastatin 20 to 40mg/d, which were considered high-intensity statin doses. Maximum combined therapy was defined as maximum statin dose plus ezetimibe 10mg/d. Maximum LLT was defined as any LLT expected to produce at least a 50% reduction in LDL-C baseline levels: simvastatin 20, 40, or 80mg/d plus ezetimibe 10mg/d; pravastatin 40mg/d in combination with ezetimibe 10mg/d; fluvastatin 80mg/d plus ezetimibe 10mg/d; atorvastatin 40 or 80mg/d with or without ezetimibe 10mg/d; atorvastatin 10 or 20mg/d plus ezetimibe 10mg/d; rosuvastatin 20 or 40mg/d with or without ezetimibe 10mg/d; rosuvastatin 10mg/d plus ezetimibe 10mg/d; and pitavastatin 4mg/d in combination with ezetimibe 10mg/d.12,13
Genetic AnalysisLow-density lipoprotein cholesterol receptor (LDLR) mutations were classified according to their known effect on LDL receptor protein function as null (receptor-negative) and defective (receptor-defective) mutations as previously described.14 Variants leading to the complete absence or truncation of the protein (loss of function) demonstrated by in vitro functional analysis or computer simulation analysis were classified as receptor-negative. These variants included the following: a) point mutations causing a premature stop codon; b) missense mutations affecting the fifth cysteine-rich repeat in the ligand-binding domain of the LDL-C receptor gene (class 2A mutation); c) small deletions or insertions causing a frame shift and a premature stop codon; and d) large rearrangements. Receptor-defective mutations were the remaining inframe point mutations and small inframe deletions and insertions. All mutations without known functionality analysis by means of in vitro studies or computer simulation analysis were classified as “unknown functionality” because we could not be certain whether the effect on the receptor was negative or defective; however, they were considered pathogenic because all individuals carrying 1 of these mutations had hypercholesterolemia, whereas relatives without the mutation had normal cholesterol levels.14
Statistical AnalysisStatistical analyses were performed using SPSS version 18.0 (SPSS Inc, Chicago, Illinois, United States). The normality of the distribution of the variables was analyzed with the Kolmogorov-Smirnov test. Quantitative data are expressed as median and interquartile range (IQR) and qualitative data as absolute number and percentage. Two populations were defined: population at entry (n = 241) and population at follow-up (otherwise known as the cohort), which included those patients who had a full plasma lipid profile at follow-up (n = 217). All comparisons between entry and follow-up were performed in the cohort study. Comparisons of frequencies between qualitative variables were performed using the chi-squared test. Changes in binary variables were analyzed by the McNemar test. Median values of quantitative variables were compared with the Mann-Whitney nonparametric test or the paired Wilcoxon signed rank test as appropriate. A forward binary logistic regression analysis was conducted in the cohort study to determine the variables associated with statin use. We included variables that were statistically significant in univariate analyses, as well as a priori predictors and confounders: age, sex, and follow-up in a primary/specialized setting. Another forward binary logistic regression analysis was conducted in the cohort study, excluding those patients who reached the goal at entry, to determine the variables associated with the attainment of LDL-C < 130mg/dL. We included variables that were statistically significant in univariate analyses, as well as a priori predictors and confounders: age, sex, type of mutation (null or defective), use of ezetimibe, and follow-up in a primary/specialized setting. Differences were considered statistically significant at P < .05.
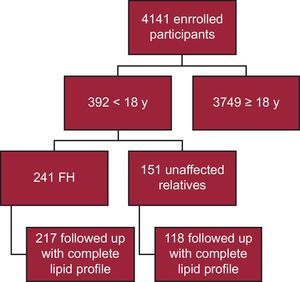
RESULTSTo date, 4141 participants have been enrolled in the SAFEHEART registry; 392 are younger than 18 years. Of these, 241 have a molecular confirmation of FH, with 217 followed up with a complete lipid profile (90.0%) (Figure 1). Twenty-four patients were omitted from the analysis due to the lack of a complete lipid profile at follow-up. Follow-up was in a primary care setting for 40 patients (18.4%). The median follow-up time was 4.69 years (IQR, 2.48-6.38 years).
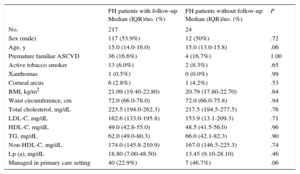
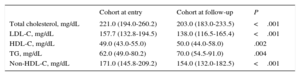
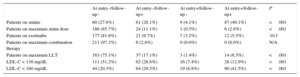
At enrollment (the at-entry population), 129 FH patients (53.5%) were male. The median age was 15.0 years (IQR, 14.0-16.0 years). The 2 youngest patients were 8 years old. History of ASCVD was not present in any patients and premature familial ASCVD was present in 40 (16.6%). Baseline characteristics are depicted in Table 1. A comparison of baseline characteristics at inclusion between cohort patients and those who were not followed up is shown in Table 1. No significant differences were found between the 2 groups. No patient had a history of ASCVD, high blood pressure, or diabetes mellitus. A higher proportion of the group without follow-up was managed in the primary care setting, although the difference was not statistically significant. In the cohort, there were significant reductions in plasma concentrations of total cholesterol, LDL-C, triglycerides, and non-HDL-C; a significant increase in HDL-C was also observed at follow-up (Table 2).
Baseline Characteristics of the At-entry Population
| FH patients with follow-up Median (IQR)/no. (%) | FH patients without follow-up Median (IQR)/no. (%) | P | |
|---|---|---|---|
| No. | 217 | 24 | |
| Sex (male) | 117 (53.9%) | 12 (50%) | .72 |
| Age, y | 15.0 (14.0-16.0) | 15.0 (13.0-15.8) | .06 |
| Premature familiar ASCVD | 36 (16.6%) | 4 (16.7%) | 1.00 |
| Active tobacco smoker | 13 (6.0%) | 2 (8.3%) | .65 |
| Xanthomas | 1 (0.5%) | 0 (0.0%) | .99 |
| Corneal arcus | 6 (2.8%) | 1 (4.2%) | .53 |
| BMI, kg/m2 | 21.09 (19.40-22.80) | 20.79 (17.80-22.70) | .64 |
| Waist circumference, cm | 72.0 (66.0-78.0) | 72.0 (66.0-75.8) | .94 |
| Total cholesterol, mg/dL | 223.5 (194.0-262.3) | 217.5 (194.5-277.5) | .76 |
| LDL-C, mg/dL | 162.6 (133.0-195.8) | 153.9 (13.1-209.3) | .71 |
| HDL-C, mg/dL | 49.0 (42.8-55.0) | 48.5 (41.5-56.0) | .96 |
| TG, mg/dL | 62.0 (49.0-80.3) | 66.0 (42.1-82.3) | .90 |
| Non-HDL-C, mg/dL | 174.0 (145.8-210.9) | 167.0 (146.5-225.3) | .74 |
| Lp (a), mg/dL | 18.80 (7.00-48.50) | 13.45 (9.10-28.10) | .46 |
| Managed in primary care setting | 40 (22.9%) | 7 (46.7%) | .06 |
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; FH, familial hypercholesterolemia; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp (a), lipoprotein (a); IQR, interquartile range; TG, triglycerides.
Plasma Lipid and Lipoprotein Concentrations (Cohort)
| Cohort at entry | Cohort at follow-up | P | |
|---|---|---|---|
| Total cholesterol, mg/dL | 221.0 (194.0-260.2) | 203.0 (183.0-233.5) | <.001 |
| LDL-C, mg/dL | 157.7 (132.8-194.5) | 138.0 (116.5-165.4) | <.001 |
| HDL-C, mg/dL | 49.0 (43.0-55.0) | 50.0 (44.0-58.0) | .002 |
| TG, mg/dL | 62.0 (49.0-80.2) | 70.0 (54.5-91.0) | .004 |
| Non-HDL-C, mg/dL | 171.0 (145.8-209.2) | 154.0 (132.0-182.5) | <.001 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides.
Values are median (interquartile range).
We identified 212 patients with a mutation in LDL-C receptor genes (97.7%) and 5 patients with a mutation in apolipoprotein B genes (2.3%). Of the mutations in LDL-C receptor genes, 95 (43.8%) were classified as null mutations, 92 (42.4%) as defective mutations, and 25 (11.5%) as unknown functionality mutations.
Lipid-lowering Therapy and Goal AttainmentTable 3 shows the use of different LLT regimens at entry and follow-up. The results show a significant increase in the use of statins (44.2% at entry and 68.2% at follow-up), ezetimibe (8.7% at entry and 15.2% at follow-up), maximum statin dose (3.3% at entry and 13.9% at follow-up), and maximum LLT (7.9% at entry and 23.6% at follow-up). The most widely prescribed statin at entry (25.3%) and follow-up (30.5%) was atorvastatin. Rosuvastatin prescription increased (from 6.0% at inclusion to 20.3% at follow-up). The median duration of statin therapy was 7.0 years (5.0 to 9.0 years). Age at menarche was 12.0 years (12.0 to 13.0 years) for girls being treated with statins and 12.0 years (11.0 to 13.0 years) for girls not being treated with statins (P = .77). No increase in either hepatic transaminases or creatine phosphokinase was observed. Fibrates and bile acid sequestrants were only used in 15 patients at inclusion (7.0%) and 3 patients at follow-up (1.5%). Ezetimibe monotherapy without a statin was used in 18 patients at inclusion (8.3%) and 24 patients at follow-up (11.1%). Regarding treatment adherence, 10 patients (4.6%) reported not taking medication at least 1 day each month during follow-up. On multivariable analysis, no variable was independently associated with statin use.
Lipid-lowering Therapies and LDL-C Goal Achievement (Cohort)
| At entry–/follow-up– | At entry–/follow-up+ | At entry+/follow-up– | At entry+/follow-up+ | P | |
|---|---|---|---|---|---|
| Patients on statins | 60 (27.6%) | 61 (28.1%) | 9 (4.1%) | 87 (40.1%) | <.001 |
| Patients on maximum statin dose | 186 (85.7%) | 24 (11.1%) | 1 (0.5%) | 6 (2.8%) | <.001 |
| Patients on ezetimibe | 177 (81.6%) | 21 (9.7%) | 7 (3.2%) | 12 (5.5%) | .013 |
| Patients on maximum combination therapy | 211 (97.2%) | 6 (2.8%) | 0 (0.0%) | 0 (0.0%) | N/A |
| Patients on maximum LLT | 163 (75.1%) | 37 (17.1%) | 3 (1.4%) | 14 (6.5%) | <.001 |
| LDL-C < 130 mg/dL | 111 (51.2%) | 62 (28.6%) | 16 (7.4%) | 28 (12.9%) | <.001 |
| LDL-C < 160 mg/dL | 44 (20.3%) | 64 (29.5%) | 19 (8.8%) | 90 (41.5%) | <.001 |
LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy.
Values are n (%). – = not present; + = present.
See text for LLT classification.
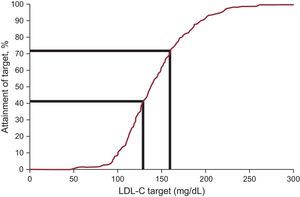
Plasma LDL-C concentration decreased by an average of 12.5%, reaching a median value of 138.0mg/dL at follow-up. Low-density lipoprotein cholesterol goals, as defined by the recent international recommendations on FH, were reached in 20.3% at entry and 41.5% at follow-up (Table 3 and Figure 2). When an alternative goal of LDL-C < 160mg/dL was considered for patients younger than 14 years, 8 out of 48 patients (16.7%) and 1 out of 6 patients (16.7%) reached the goal at inclusion and follow-up, respectively. The only variable independently associated with LDL-C goal attainment in the multivariable analysis was statin use (odds ratio, 13.83; 95% confidence interval, 2.98-64.15). The type of health care provider (specialist or primary care physician), age, sex, lipoprotein (a) level, and type of mutation were not associated with LDL-C goal attainment.
DISCUSSIONIn this study, we report the characteristics, LLT use, and LDL-C goal attainment in a longitudinal cohort of molecularly-defined FH patients younger than 18 years enrolled in the SAFEHEART registry. This unique registry of FH patients is based on data obtained from real life in Spain in both specialized and primary care settings. Our results show that an LDL-C treatment target < 130mg/dL was reached by only 20.3% of the patients at inclusion and in 41.5% at follow-up, with 68.2% of patients on LLT. Statin use was the only factor independently associated with LDL-C goal achievement. To our knowledge, no other work has shown goal attainment in FH patients younger than 18 years and this study is the first to report it in a large population.
Recently, a United Kingdom registry15 analyzed 207 children with FH, identifying mutations in 64% of children and finding that 48% were on LLT; a 35% reduction was achieved in LDL-C. However, the authors reported no goal attainment results. Another report, which analyzed a small subject sample (n = 89), showed a 43% LDL-C reduction at long-term follow-up.16 This greater reduction is probably due to a more frequent use of combined therapy (56%). No objective attainment results were shown. In another retrospective article of 207 patients in the Netherlands, only 26% of patients were on LLT and, once more, no results regarding LDL-C goal attainment were reported.17
This longitudinal study showed that LDL-C levels in FH patients younger than 18 years may change over time due to LLT modification and physician education. The proportion of patients on statins, maximum statin dose, and maximum LLT significantly increased during follow-up. Interestingly, our data indicate that our cohort is not biased because there were no statistically significant differences between the patients who were not followed up and the cohort.
Early diagnosis and management of FH is essential, particularly in children and adolescents, to prevent ASCVD development in adulthood. Screening for FH in children is worthwhile and must be carried out before the age of 8 years because children with hypercholesterolemia are at increased risk of premature ASCVD. Furthermore, screening may identify those at highest risk and prompt LLT initiation, which has been shown to effectively reverse the atherosclerotic process and reduce the ASCVD risk. Children with FH do not usually have clinical ASCVD. Nevertheless, the existence of future risk supports the use of LLT, with statins being the cornerstone of FH management.18
The safety and tolerability of LLT in pediatric FH are always controversial, although they are reported to be similar to those in adults.5,19,20 Recently, Ramaswami et al.15 reported no safety concerns, similar to our results. Nevertheless, strict supervision is recommended, especially in those patients receiving higher statin doses. Adolescent girls should also be counseled to suspend statin therapy when contemplating pregnancy. Nonetheless, although more data on safety issues for children under long-term treatment with LLT are needed, recent long-term follow-up work has shown an excellent safety profile.21 This finding is indirectly supported by our data, because a high proportion of patients initiated LLT during follow-up and there were few drop-outs. Our results clearly show an increased percentage of patients using statins, a high statin dose, and maximum LLT, with a low proportion of patients abandoning the medication. These data confirm the safety, adherence, and tolerability of statins, even when used at a high dosage, in FH patients younger than 18 years.22 Furthermore, our results agree with previous reports showing no effects on sexual maturation.23 All of these results reaffirm the concept “the younger, the better” regarding the ideal age to initiate statins in these young FH patients.23
Our results show a high number of FH patients younger than 18 years and, in accordance with previous studies,15,16 suggest the willingness of adult FH patients to include their immediate family members in screening and registry activities. This fact reflects the seriousness with which these patients take their problem and the impact that the advice of their physicians can have on changing their lifestyles. Such an attitude in adult patients constitutes the basis of a healthy lifestyle in their relatives.24
Although the most common goal for FH patients younger than 18 years is an LDL-C level below 130mg/dL, an alternative approach consisting of LDL-C < 160mg/dL may be used in those patients younger than 14 years, nonsmokers, with HDL-C ≥ 40mg/dL, lipoprotein (a) < 50mg/dL, LDL-C < 250mg/dL, and without premature cardiovascular disease in progenitors or grandparents.6 Other recent guidelines recommend a 50% reduction in LDL-C from pretreatment levels but, for those children aged ≥ 10 years, especially if there are additional cardiovascular risk factors, including elevated lipoprotein (a), the LDL-C target should be < 130mg/dL.11 Our results also show the difficulty faced by these patients of achieving lipid targets.25 Moreover, LDL-C goal achievement was similar whether patients were treated by specialists or primary care physicians. Thus, it is possible to achieve a level of care for pediatric patients with FH in a primary care setting that is comparable to that achieved by specialist care. For this goal, it is important to emphasize the support that clinicians receive via registries and dedicated training programs. Registries can optimize the management of FH patients younger than 18 years by enabling the integration of primary and specialist care and may also support health authorities in decision making.8,26
Limitations and StrengthsIn this large follow-up study of FH patients younger than 18 years, the intervention was unchanged from that provided by the patient's physician. A reliable baseline lipid profile in this registry is missing because some patients were already receiving treatment when enrolled. Furthermore, the findings may have been altered by several conditions, such as different lifestyles, and an association with different cardiovascular risk factors that could have modified the results.
CONCLUSIONSSAFEHEART registry data show that a high proportion of FH patients younger than 18 years have high LDL-C levels and fail to achieve recommended LDL-C targets. We found an increase in LLT intensity and a significant decrease in LDL-C levels during follow-up. Statin use was the only independent predictor of LDL-C goal achievement. Furthermore, no safety concerns were detected during follow-up. These results indicate that many FH patients are not adequately controlled and that there is still room for treatment improvement. Furthermore, the follow-up of this FH population may contribute to knowledge on the safety of life-long LLT and the optimal age for therapy initiation to prevent ASCVD development in adulthood.
FUNDINGThis work was supported by grant G03/181 from the Fundación Hipercolesterolemia Familiar, FIS PI12/01289 from the Instituto de Salud Carlos III, and grant 08-2008 from the Centro Nacional de Investigación Cardiovascular.
CONFLICTS OF INTERESTNone declared.
- –
Children with untreated heterozygous familial hypercholesterolemia are at increased risk of premature ASCVD after 20 years of age.
- –
Statins and other lipid-lowering therapies effectively lower LDL-C and are safe in children and adolescents.
- –
Little is known about the characteristics of FH patients younger than 18 years, the lipid-lowering therapies used in these patients, and the lipid goals reached in real life.
- –
This information deficit is even greater for follow-up data.
- –
A high proportion of FH patients younger than 18 years fail to achieve recommended LDL-C targets.
- –
We found an increase in LLT intensity and a significant decrease in LDL-C levels during follow-up.
- –
Statin use was the only independent predictor of LDL-C goal achievement and no safety concerns were detected during follow-up.
- –
These results reinforce the concept of “the younger, the better”.
The authors thank Ms. Teresa Pariente for her hard work in managing the familial cascade screening from the beginning of the SAFEHEART registry, the entire Spanish Familial Hypercholesterolemia Foundation for its assistance in the recruitment and follow-up of participants, and the FH families for their valuable contribution and willingness to participate.
Rocío Aguado (Hospital Universitario de León, León, Spain); Fátima Almagro (Hospital Donostia, Donostia-San Sebastián, Guipúzcoa, Spain); Rodrigo Alonso, Nelva Mata, Pedro Mata, Leopoldo Pérez de Isla, Adriana Saltijeral (Fundación Hipercolesterolemia Familiar, Madrid, Spain); Francisco Arrieta (Hospital Ramón y Cajal, Madrid, Spain); Lina Badimón, Teresa Padró (Instituto Catalán Ciencias Cardiovasculares, IIB-Sant Pau, Barcelona, Spain); Miguel Ángel Barba (Hospital Universitario, Albacete, Spain); Ángel Brea, Daniel Mosquera (Hospital San Pedro, Logroño, La Rioja, Spain); José María Cepeda (Hospital de Vega Baja, Orihuela, Alicante, Spain); Raimundo de Andrés (Fundación Jiménez Díaz, Madrid, Spain); Gonzalo Díaz-Soto (Hospital Clínico, Valladolid, Spain); José L. Díaz (Hospital Abente y Lago, A Coruña, Spain); Rosaura Figueras, Xavier Pintó (Hospital de Bellvitge, Barcelona, Spain); Francisco Fuentes, José López-Miranda (Hospital Reina Sofía, Córdoba, Spain); Jesús Galiana (Hospital de Ciudad Real, Ciudad Real, Spain); Juan Antonio Garrido (Hospital Arquitecto Marcide, Ferrol, A Coruña, Spain); Luis Irigoyen (Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain); Laura Manjón (Hospital de Cabueñes, Gijón, Asturias, Spain); Alberto Martín, Mar Piedecausa (Hospital General Universitario de Elche, Elche, Alicante, Spain); Ceferino Martínez-Faedo (Hospital Central de Asturias, Oviedo, Asturias, Spain); Marta Mauri (Hospital de Terrassa, Terrassa, Barcelona, Spain); Pablo Miramontes (Hospital Clínico Universitario, Salamanca, Spain); Ovidio Muñiz (Hospital Virgen del Rocío, Sevilla, Spain); Francisca Pereyra (Hospital Universitario Nuestra. Señora de Candelaria, Santa Cruz de Tenerife, Spain); Leire Pérez (Hospital Universitario Araba, Vitoria, Álava, Spain); José Miguel Pinilla (Centro de Salud San Miguel de Salinas, Alicante, Spain); Pedro Pujante (Hospital Vital Álvarez Buylla, Mieres, Asturias, Spain); Patricia Rubio, Juan Maraver, Alfredo Michan (Hospital General de Jerez de la Frontera, Jerez de la Frontera, Cádiz, Spain); Enrique Ruiz (Hospital Universitario, Burgos, Spain); Pedro Sáenz (Hospital de Mérida, Mérida, Badajoz, Spain); Juan F. Sánchez (Hospital San Pedro de Alcántara, Cáceres, Spain); José I. Vidal, Rosa Argüeso (Hospital Universitario Lucus Augusti, Lugo, Spain); Daniel Zambón (Hospital Clínic, Barcelona, Spain).