Keywords
INTRODUCTION
Since its introduction in the field of congenital heart disease, balloon angioplasty has replaced surgery as first choice in the treatment of various stenotic lesions (pulmonary and aortic stenosis, pulmonary artery stenosis, postsurgery recoarctation). However, 22 years after its initial implementation,1,2 balloon angioplasty of native coarctation continues to be a controversial technique. Following the initial enthusiasm raised by its effectiveness as described in the first clinical experiences,3,4 the use of this technique became restricted due to the reported incidence of early recoarctation5-7 and, in particular, the development of aneurysms in the angioplasty site.8-11 Despite this, several groups have continued to use balloon angioplasty as the treatment of choice for localized coarctation. Published results,12-15 along with long-term follow-up studies,16-19 show results comparable to those of surgery, as well as the absence of progression of the aneurysm in some cases. This, together with the increasingly known incidence of aortic aneurysms in coarctation patients treated surgically,20,21 has kept the debate open regarding the treatment of choice of aortic coarctation after the neonatal period.
Our experience with the percutaneous treatment of coarctation began in 198522 (3 years after the publication of the first cases); the technique was dropped in 1988 due to the observed incidence of aneurysms in our series, whose long-term prognosis was then uncertain. Five years later, in 1993, in view of the results published by groups that had continued to use this technique,12,15,17 we began to use balloon angioplasty again, initially in coarctations carrying greater surgical risk (absence of collateral circulation) and, shortly afterwards, we used it as treatment of first choice in patients older than 3 months with localized coarctation.
OBJECTIVES
The objectives of our work are: 1) to share our experience, regarding both the results and medium-term follow-up when using this technique; and 2) to compare retrospectively the results and complications of the angioplasties done in our service in the following 2 periods: between 1985 and 1988, and 1993 and 2003.
SUBJECTS AND METHODS
The clinical, hemodynamic, and echocardiographic data of pediatric patients undergoing percutaneous angioplasty of native coarctation between March 1985 and April 2003 were analyzed retrospectively. The patients were classified into 2 groups according to when angioplasty had been done: group A (patients treated between March 1985 and July 1988) and group B (patients treated between May 1993 and April 2003). Table 1 shows the data of both groups. Group A included patients older than 3 months diagnosed with coarctation with a peak pressure gradient >20 mm Hg. Group B included patients of any age with localized lesions and a pressure gradient >20 mm Hg; patients with Turner's or Noonan's syndrome were excluded.
After obtaining informed consent from relatives, catheterization was done under sedation in Group A and under general anesthesia in group B. Percutaneous access was via the femoral artery and vein in all cases, with heparinization at 100 U/kg. After angiographic study [aortography, anteroposterior (AO) and left anterior oblique (LAO) projections) and establishing the pressure gradient, a balloon was selected whose diameter was 2-4 times greater than that of the stenosis, while ensuring that it did not exceed the caliber of the diaphragmatic aorta. The balloon was introduced via an exchange guidewire placed in the ascending aorta and, after percutaneous transluminal angioplasty (PTA), this was replaced, via the guidewire, by pigtail or multipurpose catheters which were used for AP and LAO angiographies and pressure measurements. The coarctation diameters, pre- and post-angioplasty pressure gradients, and balloon-stenosis and balloon-diaphragmatic aorta relationships are shown in Table 1. In Group A arterial sheaths between 5 Fr to 11 Fr (9.1±1.29) were used, whereas in group B the sheaths were between 5 Fr and 10 Fr (6.5±1.27), thanks to the low profile of the angioplasty catheters available in that period.
All patients underwent clinical check-ups and echocardiography at follow-up. Catheterization was repeated in 13 of the 26 patients in Group A and magnetic resonance imaging (MRI) in 3. In group B new catheterization was performed in 7 patients and MRI in 10. The cumulative incidence of recoarctation or aneurysm was assessed in particular, and the possible factors involved in their appearance analyzed. The following variables were explored as patient factors: age (more than or less than 1 year), lesion and diaphragmatic aorta diameters, and pressure gradient prior to angioplasty. The following variables were explored as technique-dependent factors: immediate result (pressure gradient more than or less than 20 mm Hg), diameter of the angioplasty balloon used, balloon diameter-stenosis diameter relationship, and balloon diameter-diaphragmatic aorta diameter relationship.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation and discrete variables as absolute value and percentage. The Student's t test was used to compare continuous variables and the Fisher exact test for discrete variables. The Kaplan-Meier test and log-rank test were used to compare the time elapsed up to the appearance of recoarctation or aneurysm in the patients in group A and B. A value of P<.05 was considered statistically significant.
RESULTS
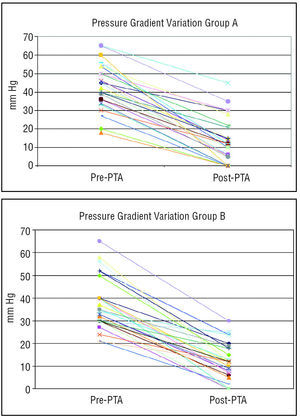
There were no significant differences between the 2 groups regarding decrease in pressure gradient (Figure 1 and Table 1) and increase in stenosis caliber (Table 1). Initially, angioplasty was successful (immediate postangioplasty peak pressure gradient <20 mm Hg) in 18 (69.2%) of 26 patients in group A versus 22 (81.5%) of 27 patients in group B (Figures 2 and 3). At follow-up, there were no significant differences in the observed incidence of recoarctation (hemodynamic or pressure gradient >20 mm Hg): 33% in group A versus 25.9% in group B. Possible factors related to the development of recoarctation were analyzed in both groups (Table 2); a greater incidence of recoarctation was found in the patients in whom angioplasty had been only partially effective (immediate postangioplasty residual pressure gradient >20 mm Hg) and in the patients with more severe coarctations (greater incidence of recoarctation to a greater pre-PTA pressure gradient, P=.015; and to a smaller pre-PTA stenosis diameter, P=.007). The incidence of recoarctation was greater in the patients younger than 1 year old, although this difference did not reach statistical significance; however, it did so with a P-value (P=.144) close to significance (Table 2). Patients with recoarctation in group A were directly referred to surgery (Figure 4), whereas a second angioplasty was done in 5 patients from group B (balloon in 3 cases and stenting in 2), with a good result (Figure 4). At the end of the follow-up period (Figure 5), in group A, 16 (62%) patients remained free from recoarctation, 7 (26.9%) had been referred to surgery due to recoarctation or aneurysm, and 1 remained with moderate recoarctation having refused surgery or new angioplasty. Out of the 27 patients in group B, 23 (85%) remained asymptomatic and free of recoarctation at the end of the follow-up period (Figure 5); only 1 (3.7%) had needed surgery (1-month-old patient at the time of angioplasty), and 3 had presented mild-moderate recoarctation.
Figure 1. Variations in the pre- and postangioplasty ascending-descending aortic peak pressure gradient (mm Hg) in group A (angioplasties done between 1985 and 1988) and group B (angioplasties done between 1993 and 2003). PTA indicates percutaneous transluminal angioplasty.
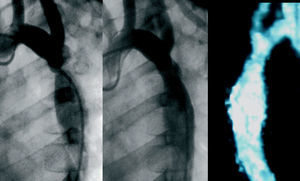
Figure 2. Six-year-old patient (group B) with severe coarctation (peak pressure gradient 40 mm Hg) and hypertension (147/95) treated with angioplasty (postangioplasty pressure gradient 7 mm Hg). Left: preangioplasty aortography, 45° left anterior oblique projection. Center: postangioplasty aortography. Right: magnetic resonance imaging done 2 years after angioplasty shows no aneurysm image; the patient presents normal blood pressure values (110/65) and absence of arm-leg pressure gradient.
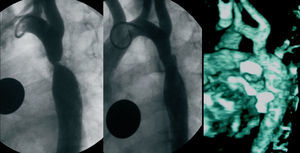
Figure 3. Five-year-old patient (group B) with severe coarctation (peak pressure gradient 50 mm Hg) and severe hypertension treated with angioplasty (postangioplasty pressure gradient 15 mm Hg). Left: preangioplasty aortography. Center: postangioplasty aortography. Right: magnetic resonance imaging done 2 years after the angioplasty, without aneurysm image.
Figure 4. Evolution during follow-up in patients in group A (angioplasties done between 1985 and 1988) and group B (angioplasties done between 1993 and 2003). PTA indicates percutaneous transluminal angioplasty.
Figure 5. Comparison of the angioplasty results in groups A and B at the end of the follow-up period.
The incidence of serious complications was greater in group A (19.2%) than in group B (3.7%), although the difference was not statistically significant. There were 5 complications in Group A: 2 deaths in the 24 h following the procedure (an aortic dissection in 1 patient with Turner's syndrome, and 1 infant with associated subaortic stenosis), 1 heart failure in the catheterization unit, and 2 arterial ischemias that required surgical thrombectomy. An acute stroke was recorded in group B which the patient recovered from with mild sequelae.
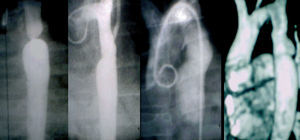
The incidence of aneurysms was also less in group B (3.7%) than in group A (15%), although the difference was not significant. In the statistical study, no patient factors (age, weight, severity of coarctation) nor angioplasty factors (balloon diameter-stenosis diameter relationship, balloon-diaphragmatic aorta relationship) were found to be related to the development of aneurysms. Of the 4 patients in group A who developed aneurysm, 2 underwent surgery because they also presented recoarctation in the other 2, we decided conservative treatment and no increase in aneurysm was observed after a follow-up time of 18 years and 15 years (Figure 6), respectively. No increase in aneurysm was found in the only patient in group B who developed this complication after a 2.5-year follow-up.
Figure 6. Patient (group A) with development of postangioplasty aneurysm. Left to right: pre-PTA angiography; immediate post-PTA angiography; angiographic control 6 years after PTA; MRI control 15 years after PTA. PTA indicates percutaneous transluminal angioplasty.
DISCUSSION
The results obtained from treating native coarctation with angioplasty in our series are comparable to the reported by other groups regarding the initial efficacy of the technique, incidence of recoarctation, and appearance of aneurysms.14,15,19,23
When comparing the results obtained in the 2 series (angioplasties performed between 1985 and 1988, and between 1993 and 2003), it is important to note that:
1. The population characteristics, the severity of coarctation, and the angioplasty technique (balloon-stenosis relationship, balloon-diaphragmatic aorta relationship) were very similar in both groups; this would explain the similarity of the initial result obtained in both series. The greater incidence of recoarctation found in the patients younger than 1 year old matches reports from other series,6,7,24 and might be accounted for by the greater amount of ductal tissue in these patients. Although the longer follow-up time in the patients in group A could act as a confounding factor when comparing the incidence of recoarctation or aneurysm between group A and B, the Kaplan-Meier analysis did not show significant differences regarding the time elapsed up to the appearance of recoarctation or aneurysm between the 2 groups (Figure 7). In fact, of the 15 patients (out of both groups) who developed recoarctation, 86.6% (13/15) did so within the first 2 years of follow-up.
Figure 7. Kaplan-Meier curves: comparison between the time elapsed until the appearance of recoarctation or aneurysm in groups A and B. NS indicates not significant.
2. Performing a second angioplasty (simple or with stent) in 5 of the cases of recoarctation in group B accounts for the better results at the end of the follow-up time in group B (85% of patients free of restenosis) than in group A (62%), despite a similar incidence of recoarctation. The use of a second angioplasty drastically changed the percentage of patients who, after percutaneous angioplasty, needed surgery: 27% in group A versus 3.7% in group B (a single patient, with neonatal coarctation). Patients who had mild to moderate pressure gradients at the end of the follow-up period undergo a new angioplasty when indicated by the severity of the injury.
3. Another important difference between the groups was the lower incidence of complications found in group B (3.7% vs 19.2% in group A), although the difference was not statistically significant due to the relatively limited number of cases in each group. This reduction is explained by the better selection of cases (e.g. excluding patients with syndromes associated with anomalies of the aortic wall, such as Turner's syndrome), the use of low-profile balloons thus reducing arterial complications, as well as optimization of the general and anesthetic care received by the patient during catheterization and in the following 24 h.
4. Regarding the observed incidence of aneurysms, and in line with other works,10 we did not find a relation between their appearance and the balloon-diaphragmatic aorta or balloon-stenosis relationship. Factors dependent on the patient and the lesion itself (presence and extension of the cystic medial necrosis8) may also influence the development of postangioplasty aneurysms. In any case, we consider that after percutaneous angioplasty of coarctation, long-term follow-up using MRI25 is fundamental to rule out the appearance of aneurysms. One of the possible limitations of our study is precisely the fact that MRI/catheterization could not be done in 100% of the patients, which means that some small aneurysms could have passed unnoticed despite x-ray and echocardiography. We do not think that the shorter follow-up time of some patients in group B accounts for the lower incidence (although insignificant) of aneurysms in group B than in group A: the Kaplan-Meier analysis did not show significant differences between the groups regarding the time elapsed until the appearance of the aneurysm (Figure 7). In fact, of the 5 patients (from both groups) who developed recoarctation, 80% (4/15) did so within the first 2 years of follow-up. Another interesting fact of our study is that the 2 patients with aneurysms who chose conservative treatment did not show significant increases in this regard at long-term follow-up (15 years), which has also been reported by other authors.11,15,19,26
The results of our study, as well as those published by other groups, lead to the following question: if, on the one hand, we are capable of effectively treating postangioplasty recoarctations percutaneously,24 and improving the results by using stents27-29 while taking into account that not all aneurysms found need surgical treatment; and, on the other hand, if the patients treated surgically also present recoarctations and can also develop aneurysms,20,21 should surgery continue to be the treatment of choice for native and localized coarctation, after the neonatal period? To answer this question we need to undertake comparative studies of the 2 techniques,30-32 preferably randomized prospective ones, with a large number of patients and long-term follow-up. These types of studies, although few,33,34 seem to indicate that the 2 techniques have comparable efficacy, with a greater incidence of recoarctation after angioplasty versus a greater incidence of serious complications34 and greater economic cost33 with surgical treatment.
Study Limitations
The relatively low number of patients in the study subtracted power from the statistical comparison; some very striking differences proved insignificant, such as the lower incidence of serious complications or of aneurysms in group B (3.7% vs 9.2% for complications, and 3.7% vs 15% for aneurysms). Although the greater follow-up time in the group A patients could act as a confounding factor when comparing the incidence of recoarctation or aneurysm between the 2 groups, the Kaplan-Meier analysis did not show significant differences between the 2 groups in the time elapsed up to the appearance of recoarctation or aneurysm (Figure 7).
CONCLUSIONS
1. Balloon angioplasty is an effective alternative treatment for native, localized aortic coarctation.
2. Better selection of patients, low-profile balloons, and optimization of general care can reduce the incidence of complications.
3. A second angioplasty (simple or with stenting) in cases of recoarctation has improved the results of this technique in the medium-long term.
ABBREVIATIONS
PTA: percutaneous transluminal angioplasty.
Fr: French.
MRI: magnetic resonance imaging.
See Editorial on Pages 1010-3
Correspondence: Dra. M.J. del Cerro Marín.
Servicio de Cardiología Pediátrica. Hospital Infantil La Paz.
Paseo de la Castellana, 261. 28046 Madrid. España.
E-mail: mcerro.hulp@salud.madrid.org
Received October 18, 2004.
Accepted for publication May 13, 2005.