Balloon pulmonary angioplasty (BPA) for inoperable chronic thromboembolic pulmonary hypertension (CTEPH) is becoming widely accepted. Procedural refinement has reduced complications. Our primary objective was to analyze the results and complications of the first national BPA program.
MethodsObservational, prospective series that included all consecutive BPA procedures in inoperable CTEPH patients between May 2013 and February 2017 performed at a single institution. We analyzed clinical and hemodynamic improvement, reperfusion pulmonary edema, and mortality.
ResultsWe performed 156 BPA sessions in 46 patients. Pulmonary vascular resistance was reduced by 44% (10.1 ± 4.9 vs 5.6 ± 2.2 WU; P < .001) and mean pulmonary arterial pressure by 23.6% (49.5 ± 12 vs 37.8 ± 9mmHg; P < .001); cardiac index rose by 17.1% (2.3 vs 2.7 L/min/m2; P = .002), N-terminal pro-B-type natriuretic peptide levels were reduced by 79.2% (1233 ± 1327 vs 255.5 ± 318 pg/dL; P < .001) and the 6-minute walk test distance improved by 74 meters (394 vs 468 m; P = .001). Reperfusion pulmonary edema developed after 9 interventions (5.8%) and 1 patient died (mortality 2.1%).
ConclusionsDue to its current refinement, BPA has become a safe and effective treatment for inoperable CTEPH that improves hemodynamics, functional status, and biomarkers with a low rate of severe periprocedural complications and mortality.
Keywords
Chronic thromboembolic pulmonary hypertension (CTEPH) causes elevated pulmonary vascular resistance (PVR) due to incomplete resolution of multiple pulmonary thromboemboli that adhere to pulmonary artery tissue and replace the normal intima, resulting in intravascular obstruction. The treatment of choice and only potentially curative strategy is pulmonary thromboendarterectomy (PTE) surgery.1 The 5-year survival of patients who undergo this operation in Spain is 86% vs 65% for those receiving medical therapy.2 Although inoperable patients receive medication for pulmonary arterial hypertension,3 the resulting functional and hemodynamic status is unsatisfactory. Balloon pulmonary angioplasty (BPA) has been proposed as a promising therapeutic alternative because published series have shown that it achieves functional, hemodynamic, and biomarker improvements in patients with pulmonary arterial hypertension.4–11
The most frequent complication and main cause of death in BPA is reperfusion pulmonary edema (RPE), which involves the appearance of pulmonary infiltrates and a saturation drop after BPA. In the first BPA series,8 61% of patients developed RPE, 3 required mechanical ventilation, and 5.5% died; accordingly, this technique was not subsequently applied to patients. Procedural refinement has reduced complications, permitting RPE rates of 0.8% to 53% (depending on the definition and diagnostic technique used) and severe RPE in just 0% to 10% of procedures.4–7,9,10
We present the first consolidated experience with this technique in a referral center for pulmonary arterial hypertension in Spain. The primary objective was to determine the clinical and hemodynamic results of BPA in patients with inoperable CTEPH. The secondary objective was to determine which variables were correlated with the development of RPE in this series, which incorporates the recommended measures to minimize this complication.
METHODSStudy PatientsThe BPA program began in May 2013. Study candidates comprised nonsurgical patients followed up in the unit and patients referred for re-evaluation from May 2013. The therapeutic decision was made in a multidisciplinary CTEPH session; PTE was evaluated as the first option. If patients’ comorbidities, distal damage, or personal preferences led to rejection of PTE, BPA was considered if the lesions were suitable for percutaneous treatment. The inclusion criteria for the BPA program were as follows: a) patients in functional class III or IV receiving optimal medical therapy, and b) patients in functional class II receiving medical therapy with intermediate- or high-risk prognostic criteria according to the European guidelines for pulmonary hypertension.3 Patients who did not meet either of these criteria continued with medical therapy. The exclusion criteria were as follows: a) absence of candidate lesions for BPA; b) asymptomatic patients or those in functional class II without intermediate- or high-risk prognostic criteria; and c) refusal to sign informed consent. The protocol was approved by the institutional ethics committee. All patients signed informed consent forms before each procedure.
Medical Therapy Prior to Balloon Pulmonary AngioplastyAll patients were anticoagulated and received oxygen therapy or diuretics as needed and specific medication for pulmonary arterial hypertension: endothelin receptor antagonists, phosphodiesterase 5 inhibitors, prostanoids, and riociguat. Patients with high-risk prognostic criteria (signs or symptoms of right heart failure, syncope, World Health Organization [WHO] class IV, cardiac index < 2 L/min/m2, or N-terminal pro-B-type natriuretic peptide > 1400 pg/dL)3 were initiated on intravenous epoprostenol 48hours before the first BPA procedure, which was maintained for the first 2 procedures during hospitalization and stopped before discharge if the prognostic criteria had improved to intermediate or low risk.4,6
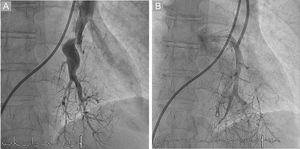
Balloon Pulmonary Angioplasty ProcedureWebs, bands, ring-like stenoses, and pouch lesions were treated to enable distal passage of contrast agent in the late phase.4,6,7,10 All procedures were performed by 2 senior interventional cardiologists with experience in the treatment of patients with pulmonary hypertension and with knowledge of the pulmonary vascular tree; the patient was awake and without sedation. The procedures were performed according to the standard refined technique,4,7,10,11 limited to 2 to 3 segmental branches of a single lobe per procedure as long as the mean pulmonary arterial pressure (mPAP) was > 35mmHg.4,5,8,11 The intervention included the treatment of all treatable lesions in all lobes until normalization of hemodynamic parameters and functional class; thus, the program comprised several sessions, with cumulative benefit obtained from each procedure. Only 1 lobe was usually treated per session to avoid the development of RPE, and 5 or 6 procedures were necessary per patient. The intervention was begun at 1 of the 2 lower lobes (generally the most damaged lobe) because they have the highest amount of lung tissue. The most commonly used guide catheter was a multipurpose catheter, followed by a JR 4. Also used were 0.014-inch, polymeric, low-tip-load, Pilot 50 or Whisper coronary angioplasty guide wires, due to their ability to easily penetrate intraluminal webs. Dilatation began with small balloons, followed by balloons of increasing sizes up to a maximum ≤ 80% of the vessel size to avoid damage to the vessel wall and its potential rupture. Vessel size was estimated visually via angiography. Dilatation was considered effective when the distal flow improved, in addition to increased contrast uptake in the lung tissue distal to the treated area and improved pulmonary venous return to the left atrium4,7 (Figure 1). A pressure guide wire was used from September 2015 in 82 of the 156 procedures to ensure the mPAP distal to the treated areas was < 35mmHg in order to avoid RPE development. The reasons for procedure completion were a peripheral arterial oxygen saturation drop > 4%, hemoptysis, treatment of the maximum number of segmental branches, or risk of contrast agent-associated nephrotoxicity.4 The procedures were repeated every 6 to 12 weeks until all candidate lesions were treated4–7 as long as the mPAP prior to BPA was > 25mmHg and the PVR was > 3 WU. The criteria for BPA program interruption were as follows: a) absence of improvement of at least 1 grade in the WHO functional class or a PVR reduction > 20% after 3 BPA procedures, and b) frail or elderly patients unable to tolerate the procedures due to their duration and the patients’ prolonged immobilization. The criterion for treatment interruption was typical and based on experience, after the observation that, with 3 procedures, the patients experienced a significant clinical and hemodynamic improvement, with a mean PVR reduction ≥ 35%.
Postprocedural Management of Balloon Pulmonary AngioplastyThe development of RPE or pulmonary hemorrhage was monitored at 48hours after the procedure. Clinical RPE was defined as a persistent fall in peripheral arterial oxygen saturation after BPA and pulmonary infiltrates on chest radiography in the treated lobes.4 When RPE developed, it was treated according to a previously established protocol.7,12 In all patients with RPE, a negative fluid balance was induced via diuretics and the oxygen supply was increased. For patients with severe RPE, if this approach did not achieve adequate oxygenation, extracorporeal membrane oxygenation was initiated. If poor oxygenation persisted, mechanical ventilation was applied.7,10,12 Hemodynamic parameters were determined before each BPA procedure and 6 months after program completion. Biochemical parameters, the 6-minute walk test, and functional class were evaluated after the third BPA session, at the end of the program, and at 6 months. Once the treatment was completed, the specific medication for pulmonary arterial hypertension was gradually reduced in patients with low-risk prognostic criteria (WHO I-II, no signs or symptoms of right heart failure, cardiac index ≥ 2.5 L/min/m2, and N-terminal pro-B-type natriuretic peptide < 300 pg/dL),3 mPAP < 30mmHg, and PVR < 4 WU.
Analysis of Treatment OutcomesThe efficacy results of the technique were analyzed in the entire population as a whole and specifically in patients who underwent at least 3 procedures; the cumulative hemodynamic benefit of the sum of the procedures was determined, as well as functional and biomarker improvements after the third BPA procedure.
Statistical AnalysisAll analyses were performed with the statistical program STATA 12.1. Categorical variables are presented as absolute frequency and proportions, and continuous variables as the mean ± standard deviation. The Kolmogorov-Smirnov test was used to test the normality of the distribution. The Student t test was used for the comparative study of the continuous variables; if the variables did not follow a normal distribution, the Wilcoxon signed-rank test was used. The percentages were compared by means of the chi-square test, Fisher exact test, or Wilcoxon test, as appropriate. A multivariate analysis (logistic regression) was performed to identify the variables associated with RPE. The inclusion criteria were clinical relevance and biological plausibility. The Mickey criteria were used to exclude variables with P > .20 in the univariate analysis. P < .05 was considered statistically significant.
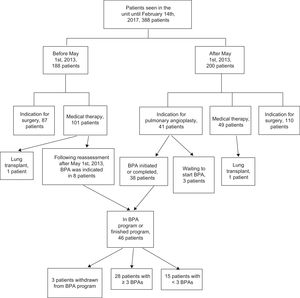
RESULTSUp to February 2017, 156 BPA sessions were performed in 46 patients with CTEPH; 28 underwent at least 3 procedures. The patient flow diagram is shown in Figure 2. Since BPA became available, the new patients who underwent the various treatment possibilities were as follows: PTE at 55.0%, BPA at 20.5%, and medical therapy at 24.5%. Of the 46 patients, 16 completed the BPA program, with an average of 4.8 (3-9) sessions per patient.
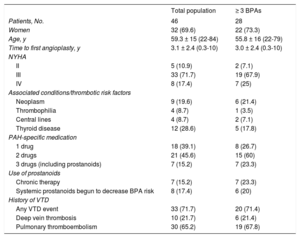
Baseline CharacteristicsThe baseline characteristics of the 46 patients and the 28 who underwent at least 3 procedures are described in Table 1. Pulmonary thromboendarterectomy was ruled out due to a distal lesion location in all patients except 3. These 3 patients showed damage to the origin of the segmental branches: 1 had syringomyelia with severe ventilatory restriction and the other 2 refused the operation. In 3 of the 46 patients, the treatment was discontinued, 2 due to a lack of improvement (patient ages of 79 and 78 years) and 1 (82 years) due to poor tolerance of the lengthy procedures and wheelchair confinement due to severe osteoarthritis.
Baseline Characteristics of the Study Population
| Total population | ≥ 3 BPAs | |
|---|---|---|
| Patients, No. | 46 | 28 |
| Women | 32 (69.6) | 22 (73.3) |
| Age, y | 59.3 ± 15 (22-84) | 55.8 ± 16 (22-79) |
| Time to first angioplasty, y | 3.1 ± 2.4 (0.3-10) | 3.0 ± 2.4 (0.3-10) |
| NYHA | ||
| II | 5 (10.9) | 2 (7.1) |
| III | 33 (71.7) | 19 (67.9) |
| IV | 8 (17.4) | 7 (25) |
| Associated conditions/thrombotic risk factors | ||
| Neoplasm | 9 (19.6) | 6 (21.4) |
| Thrombophilia | 4 (8.7) | 1 (3.5) |
| Central lines | 4 (8.7) | 2 (7.1) |
| Thyroid disease | 12 (28.6) | 5 (17.8) |
| PAH-specific medication | ||
| 1 drug | 18 (39.1) | 8 (26.7) |
| 2 drugs | 21 (45.6) | 15 (60) |
| 3 drugs (including prostanoids) | 7 (15.2) | 7 (23.3) |
| Use of prostanoids | ||
| Chronic therapy | 7 (15.2) | 7 (23.3) |
| Systemic prostanoids begun to decrease BPA risk | 8 (17.4) | 6 (20) |
| History of VTD | ||
| Any VTD event | 33 (71.7) | 20 (71.4) |
| Deep vein thrombosis | 10 (21.7) | 6 (21.4) |
| Pulmonary thromboembolism | 30 (65.2) | 19 (67.8) |
BPA, balloon pulmonary angioplasty; NYHA: New York Heart Association; PAH, pulmonary arterial hypertension; VTD, venous thromboembolic disease.
Values represent No. (%) or mean ± standard deviation (range).
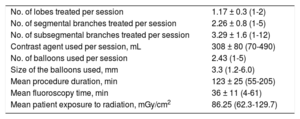
Information on the BPA procedures is shown in Table 2.
Characteristics of the 156 Balloon Pulmonary Angioplasty Procedures
| No. of lobes treated per session | 1.17 ± 0.3 (1-2) |
| No. of segmental branches treated per session | 2.26 ± 0.8 (1-5) |
| No. of subsegmental branches treated per session | 3.29 ± 1.6 (1-12) |
| Contrast agent used per session, mL | 308 ± 80 (70-490) |
| No. of balloons used per session | 2.43 (1-5) |
| Size of the balloons used, mm | 3.3 (1.2-6.0) |
| Mean procedure duration, min | 123 ± 25 (55-205) |
| Mean fluoroscopy time, min | 36 ± 11 (4-61) |
| Mean patient exposure to radiation, mGy/cm2 | 86.25 (62.3-129.7) |
Values represent the mean (range) or mean ± standard deviation (range).
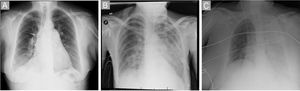
Complications are shown in Table 3. Mortality is calculated by patient but the remaining complications are detailed by procedure. Intraprocedural hemoptysis/hemosputum was the most frequent complication. None progressed to RPE, and all were resolved by anticoagulant reversal and procedure interruption. The second most common complication was RPE: 6 developed after the first BPA session and 3 after the second. Seven were subclinical, of grade 2 or 3 (Figure 3A), 1 was grade 1 (Figure 3B), and 1 was grade 5 (Figure 3C); the latter patient required mechanical ventilation and venoarterial extracorporeal membrane oxygenation. The patient died at 8 days due to cerebral hemorrhage (2.1% mortality in this series). This patient had severe hemodynamic deterioration, was in functional class IV despite triple combination therapy, and had a pre-BPA mPAP of 69mmHg. The RPE occurred after the first BPA procedure. One patient required pericardium-covered stent implantation due to guidewire perforation.
Balloon Pulmonary Angioplasty Complications
| No complications | 112 (71.8%) |
| Hemoptoic sputum | 20 (12.8%) |
| Reperfusion edema | 9 (5.8%) |
| Severe | 1 (0.6%) |
| Death | 1 (2.1%) |
| Catheter dissection without flow compromise | 10 (6.4%) |
| Perforation requiring an intervention | 1 (0.6%) |
| Extravasation not requiring an intervention | 2 (1.3%) |
| Guidewire or balloon dissection | 1 (0.6%) |
| Femoral hematoma requiring blood transfusion | 1 (0.6%) |
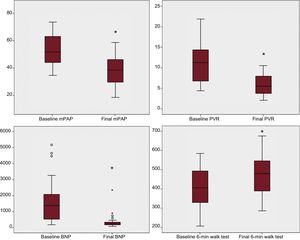
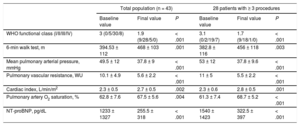
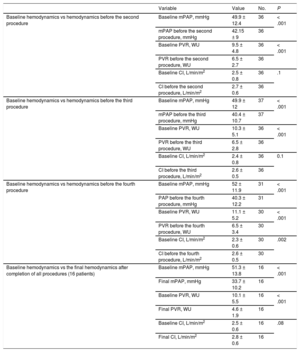
The outcomes were obtained with a mean follow-up of 15.3 ± 9 (1-46) months for the 43 patients in the BPA program and with a mean follow-up of 19 ± 9 (5-46) months for the 28 patients with at least 3 BPA procedures (61% of the total). The 43 patients achieved a significant improvements in function (6-minute walk test and WHO functional class) and hemodynamic and biomarker parameters, improvements that were more marked in the group of 28 patients who underwent at least 3 procedures (Table 4, Figure 4, and Figure 5). The main changes in the total population were as follows: increase of 74 meters in the distance covered in the 6-minute walk test, a 44% reduction in the PVR, and a 79% reduction in the N-terminal pro-B-type natriuretic peptide fraction. In the group of 28 patients with at least 3 procedures, the PVR reduction was 50%. The progressive and cumulative hemodynamic improvement with the BPA program is shown in Table 5. One patient who completed the treatment continued to have high-risk prognostic criteria and is currently on the lung transplant waiting list.
Changes in Hemodynamic, Functional, and Biomarker Parameters During Follow-up in the Total Population and the 28 Patients With at Least 3 Procedures
| Total population (n = 43) | 28 patients with ≥ 3 procedures | |||||
|---|---|---|---|---|---|---|
| Baseline value | Final value | P | Baseline value | Final value | P | |
| WHO functional class (I/II/III/IV) | 3 (0/5/30/8) | 1.9 (9/28/5/0) | < .001 | 3.1 (0/2/19/7) | 1.7 (9/18/1/0) | < .001 |
| 6-min walk test, m | 394.53 ± 112 | 468 ± 103 | .001 | 382.8 ± 116 | 456 ± 118 | .003 |
| Mean pulmonary arterial pressure, mmHg | 49.5 ± 12 | 37.8 ± 9 | < .001 | 53 ± 12 | 37.8 ± 9.6 | < .001 |
| Pulmonary vascular resistance, WU | 10.1 ± 4.9 | 5.6 ± 2.2 | < .001 | 11 ± 5 | 5.5 ± 2.2 | < .001 |
| Cardiac index, L/min/m2 | 2.3 ± 0.5 | 2.7 ± 0.5 | .002 | 2.3 ± 0.6 | 2.8 ± 0.5 | .001 |
| Pulmonary artery O2 saturation, % | 62.8 ± 7.6 | 67.5 ± 5.6 | .004 | 61.3 ± 7.4 | 68.7 ± 5.2 | < .001 |
| NT-proBNP, pg/dL | 1233 ± 1327 | 255.5 ± 318 | < .001 | 1540 ± 1423 | 322.5 ± 397 | < .001 |
NT-proBNP, N-terminal pro-B-type natriuretic peptide; WHO, World Health Organization.
Changes in hemodynamic, biomarker, and functional parameters in patients who underwent at least 3 procedures, shown as a box plot distribution. BNP, pro-B-type natriuretic peptide; mPAP: mean pulmonary arterial pressure; PVR, pulmonary vascular resistance. *Statistically significant.
Progressive and Cumulative Improvement in Hemodynamic Parameters After Multiple Procedures
| Variable | Value | No. | P | |
|---|---|---|---|---|
| Baseline hemodynamics vs hemodynamics before the second procedure | Baseline mPAP, mmHg | 49.9 ± 12.4 | 36 | < .001 |
| mPAP before the second procedure, mmHg | 42.15 ± 9 | 36 | ||
| Baseline PVR, WU | 9.5 ± 4.8 | 36 | < .001 | |
| PVR before the second procedure, WU | 6.5 ± 2.7 | 36 | ||
| Baseline CI, L/min/m2 | 2.5 ± 0.8 | 36 | .1 | |
| CI before the second procedure, L/min/m2 | 2.7 ± 0.6 | 36 | ||
| Baseline hemodynamics vs hemodynamics before the third procedure | Baseline mPAP, mmHg | 49.9 ± 12 | 37 | < .001 |
| mPAP before the third procedure, mmHg | 40.4 ± 10.7 | 37 | ||
| Baseline PVR, WU | 10.3 ± 5.1 | 36 | < .001 | |
| PVR before the third procedure, WU | 6.5 ± 2.8 | 36 | ||
| Baseline CI, L/min/m2 | 2.4 ± 0.8 | 36 | 0.1 | |
| CI before the third procedure, L/min/m2 | 2.6 ± 0.5 | 36 | ||
| Baseline hemodynamics vs hemodynamics before the fourth procedure | Baseline mPAP, mmHg | 52 ± 11.9 | 31 | < .001 |
| PAP before the fourth procedure, mmHg | 40.3 ± 12.2 | 31 | ||
| Baseline PVR, WU | 11.1 ± 5.2 | 30 | < .001 | |
| PVR before the fourth procedure, WU | 6.5 ± 3.4 | 30 | ||
| Baseline CI, L/min/m2 | 2.3 ± 0.6 | 30 | .002 | |
| CI before the fourth procedure, L/min/m2 | 2.6 ± 0.5 | 30 | ||
| Baseline hemodynamics vs the final hemodynamics after completion of all procedures (16 patients) | Baseline mPAP, mmHg | 51.3 ± 13.8 | 16 | < .001 |
| Final mPAP, mmHg | 33.7 ± 10.2 | 16 | ||
| Baseline PVR, WU | 10.1 ± 5.5 | 16 | < .001 | |
| Final PVR, WU | 4.6 ± 1.9 | 16 | ||
| Baseline CI, L/min/m2 | 2.5 ± 0.6 | 16 | .08 | |
| Final CI, L/min/m2 | 2.8 ± 0.6 | 16 |
CI, cardiac index; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance.
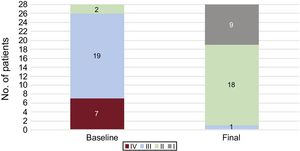
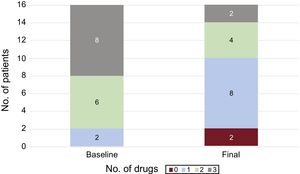
Of the 43 patients, 16 had completed the treatment at the time of article submission. In 10 of the 16 patients, the specific medication for pulmonary hypertension was reduced. All 3 drugs were withdrawn from 1 patient, as well as 2 drugs from 2 patients and 1 drug from 7 patients (Figure 6). Patients still undergoing BPA are taking the same medication.
Factors Predicting the Development of Reperfusion EdemaEight variables were chosen that a priori could be involved in RPE development to identify possible correlations: age, sex, baseline mPAP and PVR, number of lobes and segmental branches treated in each procedure, prostanoid treatment, and the patient's first procedure. In the multivariate analysis, the first BPA procedure was correlated with RPE development (odds ratio = 5.92, 95% confidence interval, 1.09-32.25, P = .04). Preprocedural mPAP showed a nonsignificant tendency to be associated with RPE (odds ratio = 2.99, 95% confidence interval, 0.91-9.84, P = .072).
DISCUSSIONIn the present series, BPA improved functional capacity (functional class and 6-minute walk test), the hemodynamic profile, and biomarkers in patients with inoperable CTEPH. The rates of severe complications and mortality were low. In addition, the progressive improvement in patients’ risk profiles allowed the specific coadjuvant medical therapy to be reduced. This work also provides information on the current treatment of these patients in a referral center for CTEPH.
Study PopulationThe population in question is considered severely ill, with one of the worst clinical and hemodynamic profiles.4,5,9,13 The time from diagnosis to first angioplasty was long in 2 Japanese series4,6 and shorter in a European series.9 Female sex predominates, as noted in the bibliography, and the age range of patients covers more extreme ages and includes patients in the third decade of life (9%) and a significant number of people older than 70 years (24%).4,5,9
Since the publication of the results of the Japanese series4,6,7 and the class IIb recommendation for BPA in the 2015 European guidelines,3 numerous centers in Japan and Europe have begun BPA programs.9,10,12,14 However, there is no information on the percentage of CTEPH patients who undergo this treatment in clinical practice. This figure is unknown in both the Japanese series, which performed considerably fewer PTEs than BPAs,4,7,12 and in European series, which limited the BPA to referral centers with established PTE programs4,9 that performed more operations than BPAs. According to the Spanish registry of pulmonary hypertension,1 the PTE rate is 31.2% in Spain but reaches 48% in expert CTEPH centers. Since BPA was introduced, the technique has been indicated to 20.5% of new patients. Currently, in our setting and in a referral unit, BPA plays an important role as a coadjuvant to medical therapy for 20.5% of patients with CTEPH and 45.5% of poor candidates for PTE.
Balloon Pulmonary Angioplasty OutcomesThe present work confirms that the significant clinical, hemodynamic, and biomarker benefits of BPA surpass those of medical therapy,4,11,15 benefits that are even more marked after 3 or more BPA procedures. When the entire population is analyzed as a whole (as in published series),4–6,9 and more markedly if patients with at least 3 procedures are specifically analyzed, the hemodynamic and functional improvement in this series approximates that of the Japanese series (PVR reduction of 45%-69%4,6,7 and an increase > 70 meters in the 6-minute walk test)4,6 and exceeds the results of the European series (PVR reduction of 26% to 33% and an increase of just 33 meters in the 6-minute walk distance).5,9 Various rationales have been advanced to explain the differences in the improvement between the European and Japanese series; these include greater experience with BPA and management of patients with proximal lesions in Japanese centers; such patients would have undergone an operation in European referral centers but would have achieved greater hemodynamic improvement after BPA upon treatment of proximal disease.9,13,16 However, in the present series, the results obtained are in line with those of the Japanese series, despite the treatment of patients with distal damage in segmental and subsegmental branches. Our results can possibly be explained by performance of the procedures according to the refined standardized technique described in the Japanese series,4 the concentration of procedures and experience in the technique in a single center,17 our status as a national referral center for pulmonary hypertension with expert diagnosis and management of these patients, a detailed understanding of the pulmonary tree with 150 diagnostic pulmonary arteriograms performed before BPA introduction, a higher number of segmental and subsegmental branches treated per procedure vs the other European series (2.26 ± 0.8 segmental branches and 3.29 ± 1.6 subsegmental branches per session in this series vs 2 vessels/procedure in the German series),9 and the use of larger balloons (up to 6mm in this series vs 4mm in the German series).9,16
Another benefit of BPA is the decrease in specific medication needs.4 In the present series, specific medication use was reduced in 62% of completely treated patients, including systemic prostanoids. This has important economic implications and improves patients’ quality of life.
Complications: Reperfusion Edema and MortalityWe must distinguish between mild, asymptomatic, or paucisymptomatic RPE, which only requires diuretics and oxygen supplementation, and severe RPE, which requires invasive assistance. Mild RPE has moderate frequency in all series, and severe RPE is much less frequent. In both cases, the present series compares favorably with the medical literature, with a general postprocedural rate of RPE of 5.8% and a mechanical ventilation rate of 2.1% (constituting 1 patient with severe RPE). In Japanese series such as that of Mizoguchi et al.,4 RPE developed in 60% of the procedures, and mechanical ventilation was required for 5.8% of patients, whereas in that of Inami et al.,7 RPE developed in 38%, with a severe RPE rate of 6.4%, and 0.7% of patients required extracorporeal membrane oxygenation. In European series, the postprocedural RPE rate ranged from 1.8%9 to 9.5%,5 and mechanical ventilation was required in either 1 patient (5%)5 or none.9 The low RPE rate in the present series could be explained by our use of a refined technique and the treatment of a maximum of 3 segmental branches of a single lobe as long as the mPAP was > 35mmHg.
Similarly, the mortality rate was very low, at 2.1%, lower than that of some European series5,18 (10% mortality in both), similar to that recently published by a German group (1.8% mortality),9 and similar to that of recent Japanese series, between 1.5%4,7 and 3.4%.6,13,14 In our opinion, the low mortality rate can be explained by the very low rates of RPE and perforations requiring an intervention (the main causes of mortality), as well as a precise knowledge of the adequate management of the complications inherent to this technique.19
Variables Correlated With Reperfusion Pulmonary Edema DevelopmentGiven that RPE is one of the 2 main causes of death of this procedure, prediction of the variables associated with its development is considered important. The first BPA procedure was correlated with RPE development while preprocedural mPAP showed a tendency to be associated with RPE. According to some authors, RPE is triggered by sudden exposure to an elevated distal perfusion pressure, after dilatation of the stenoses protecting the distal territories.4,7 This would explain why RPE is more likely after the first procedure (when pulmonary pressure is higher) and in procedures with higher mPAP, as in the present series. Similarly, in the series by Feinstein et al.,8 RPE was correlated with a mPAP > 35mmHg and, in that by Inami et al.,7 RPE was correlated with PVR, mPAP, and PEPSI score, which considers the number of branches treated, the change in the degree of perfusion in the treated branches, and the PVR value.
LimitationsBecause the numbers of patients in this series are low and the follow-up time is short, larger series with longer follow-up times are necessary to confirm that these results are maintained. In addition, there is still no information on survival benefits with this technique due to the lack of long-term follow-up. Finally, randomized multicenter studies are required that compare the results of this treatment with medical therapy in inoperable patients.
CONCLUSIONSBy improving functional capacity and hemodynamic and biomarker parameters and decreasing the need for specific medication for pulmonary arterial hypertension, BPA performed in an expert center continues to become established as an effective therapeutic tool as an adjuvant to medical therapy in a significant percentage of patients with inoperable CTEPH. Procedural refinement has greatly increased the safety profile of BPA, achieving very low rates of RPE and mortality in expert centers, which confers the technique with a very favorable risk-benefit ratio for the patient. Reperfusion pulmonary edema remains one of the causes of mortality of this technique. The first BPA procedure and an elevated mPAP increase the likelihood of postprocedural RPE development.
FUNDINGThis study was funded by the Instituto de Salud Carlos III, Spanish Ministry of Economy, Industry, and Competitiveness, through the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (Biomedical Research Networking Center on Cardiovascular Diseases; CB16/11/00502).
CONFLICTS OF INTERESTNone declared.
- –
Balloon pulmonary angioplasty is an emerging therapeutic option for patients with inoperable CTEPH. Evidence from observational studies supports the effectiveness of this technique in improving the hemodynamics and exercise capacity of patients with CTEPH. There is a discrepancy in the strength of the outcomes between the European centers performing this technique and the Japanese series. Because of the not insignificant rate of severe complications inherent to this technique, it should be carried out in centers with expertise in its performance and in the management of patients with pulmonary hypertension.
- –
This is the first series describing the Spanish experience with BPA. It is also the first series to describe the centralized experience of a national referral center with this therapeutic technique for pulmonary hypertension. The therapeutic effectiveness and complication results obtained position BPA as a coadjuvant to effective medical therapy with a very favorable risk-benefit ratio in European centers. In addition, this series provides new information on the role currently played by BPA in a referral unit as one of the therapeutic options for patients with CTEPH.
.