Patients older than 75 years with ST-segment elevation myocardial infarction undergoing primary angioplasty in cardiogenic shock have high mortality. Identification of preprocedural predictors of short- and long-term mortality could be useful to guide decision-making and further interventions.
MethodsWe analyzed a nationwide registry of primary angioplasty in the elderly (ESTROFA MI+75) comprising 3576 patients. The characteristics and outcomes of the subgroup of patients in cardiogenic shock were analyzed to identify associated factors and prognostic predictors in order to derive a baseline risk prediction score for 1-year mortality. The score was validated in an independent cohort.
ResultsA total of 332 patients were included. Baseline independent predictors of mortality were anterior myocardial infarction (HR 2.8, 95%CI, 1.4-6.0 P=.005), ejection fraction<40% (HR 2.3, 95%CI, 1.14-4.50 P=.018), and time from symptom onset to angioplasty >6hours (HR 3.2, 95%CI, 1.6-7.5; P=.001). A score was designed that included these predictive factors (score “6-ANT-40”). Survival at 1 year was 54.5% for patients with score 0, 32.3% for score 1, 27.4% for score 2 and 17% for score 3 (P=.004, c-statistic 0.70). The score was validated in an independent cohort of 124 patients, showing 1-year survival rates of 64.5%, 40.0%, 28.9%, and 22.2%, respectively (P=.008, c-statistic 0.68).
ConclusionsA preprocedural score based on 3 simple clinical variables (anterior location, ejection fraction<40%, and delay time >6 hours) may be used to estimate survival after primary angioplasty in elderly patients with cardiogenic shock and to guide preinterventional decision-making.
Keywords
Due to population aging in western countries, there is a progressive increase in the proportion of patients admitted to hospital with a diagnosis of ST-segment elevation myocardial infarction (STEMI).1 Primary angioplasty is the treatment of choice for reperfusion when it can be performed in a timely fashion. Although advanced age is associated with a worse prognosis after STEMI, primary angioplasty is still the preferred reperfusion strategy in these patients.2,3
Cardiogenic shock (CS) is the most important complication in STEMI patients. The incidence of CS is around 6% and short-term mortality is commonly reported to be 45% to 50%.4,5 In the elderly population (> 75 years), the incidence is around 10% in recently published large registries.6,7 This condition dramatically increases short-term mortality in these patients.8–11 The survival benefit of revascularization in patients >75 years in CS has been questioned since the publication of a prespecified subgroup analysis of the SHOCK trial.12 Subsequent studies, including the SHOCK registry, have found a benefit for percutaneous coronary intervention (PCI) in these patients but the magnitude of this benefit is still debated.10,13–16
There is a scarcity of information on predictors of mortality in elderly patients with CS complicating STEMI and undergoing primary angioplasty. PCI failure, final Thrombolysis in Myocardial Infarction (TIMI) grade flow 0 to 2 and multivessel disease have been identified,11,16 all of these factors being unknown before PCI. These analyses were conducted in small series of patients and were thus unable to identify baseline (pre-PCI) predictors of outcome. Given the very high mortality observed in elderly patients undergoing PCI in CS, the identification of baseline preprocedural predictors of short- and long-term mortality could be especially useful to guide decision-making and further interventions.
In this study, we sought to describe the baseline characteristics, management, outcomes, and prognostic predictors in elderly patients admitted with CS complicating STEMI and undergoing primary PCI under current standards.
METHODSThis analysis was conducted in a nationwide registry focused on elderly patients with STEMI undergoing primary angioplasty in 31 centers throughout Spain.7 This registry is supported by the Interventional Cardiology Working Group of the Spanish Society of Cardiology and is part of the ESTROFA (Estudio Español Sobre Trombosis de Stents Farmacoactivos [Spanish Thrombosis in Drug-eluting Stents Study]) study group.
PopulationEach participating center retrospectively enrolled a strictly consecutive series of patients aged >75 years who underwent PCI due to STEMI within 24hours of pain onset. No clinical or angiographic exclusion criteria were applied. The sample size of the series of each center depended on the number of admissions in patients with STEMI to that center. Only patients with at least 1 year of follow-up from the starting date of enrollment were included. The inclusion period began in 2006 (at the earliest) and ended in 2013, although most patients were enrolled from 2010 to 2013. For the purpose of the present analysis, the subgroup of patients with CS at the time of the PCI procedure were identified.
A score-validation cohort was selected in a subsequent period (2014-2016). This cohort comprised consecutive elderly patients undergoing primary PCI in CS.
The procedures were performed according to the preferences of each operator and each institution. The clinical, angiographic, procedural, and follow-up information was collected in a specifically designed study database. The corresponding author had full access to all the data in the study and takes responsibility for its integrity and the data analysis. Data anonymity was strictly guaranteed.
CS was defined as systolic blood pressure <90mmHg for >30minutes or requiring inotropes to maintain systolic blood pressure >90mmHg and not due to vagal reaction, evidence of low cardiac output, end-organ hypoperfusion (eg, resting tachycardia, oliguria, cold extremities, or altered mental status), and/or elevated filling pressures (eg, pulmonary congestion on examination or chest X-ray).
Left ventricular ejection fraction (LVEF) was reported from the very first assessment, which always corresponded to the periprocedural period, mostly prior to PCI and sometimes during or within the first 60minutes after PCI.
The primary endpoint in this study was all-cause mortality. The survival status of patients was assessed by the investigators by means of medical records, reports and databases and through direct contact with patients and/or their relatives.
Statistical AnalysisContinuous variables are presented as mean ±standard deviation. Categorical variables are expressed as percentages. Continuous variables were compared with the t test if they followed a normal distribution or with the Wilcoxon test when not normally distributed (distribution type was assessed with the Kolmogorov-Smirnov test). Categorical variables were compared with the chi-square test or the Fischer exact test, as required. Kaplan-Meier curves of event-free survival were obtained for each prespecified group or subgroup and were compared using the log-rank test. A logistic regression analysis was conducted to establish factors independently associated with CS. We used Cox proportional-hazards regression to determine hazard ratios for 1-year mortality in the analyzed subgroups and to identify independent predictors of mortality. The centers were included in the analysis and were categorized in groups according to their 1-year mortality rates. A predefined cutoff value of longer than 6hours was selected for delay times and a value of less than 40% for severe LVEF depression. The c-statistic was obtained from the receiver operating characteristic for the combinations of predictive variables and the derived predictive score. An independent validation was obtained in a population separate from that used to develop the model. The model included all variables showing an association with the incidence of 1-year mortality in the univariate analysis (P <.2). A P value <.05 was considered statistically significant. All statistical analyses were performed using SPSS version 19 for windows.
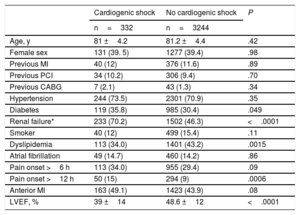
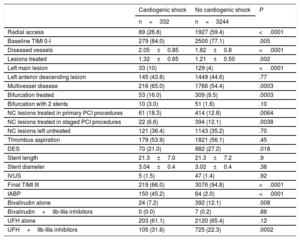
RESULTSA total of 3576 patients were included in 31 centers and, among these, 332 were in CS at the time of PCI. The clinical characteristics of patients with and without CS are shown in Table 1. The former were more frequently diabetic, were more likely to have renal failure and lower LVEF, and showed a trend for more anterior infarction and longer delay times at presentation.
Clinical Characteristics
| Cardiogenic shock | No cardiogenic shock | P | |
|---|---|---|---|
| n=332 | n=3244 | ||
| Age, y | 81 ±4.2 | 81.2 ±4.4 | .42 |
| Female sex | 131 (39. 5) | 1277 (39.4) | .98 |
| Previous MI | 40 (12) | 376 (11.6) | .89 |
| Previous PCI | 34 (10.2) | 306 (9.4) | .70 |
| Previous CABG | 7 (2.1) | 43 (1.3) | .34 |
| Hypertension | 244 (73.5) | 2301 (70.9) | .35 |
| Diabetes | 119 (35.8) | 985 (30.4) | .049 |
| Renal failure* | 233 (70.2) | 1502 (46.3) | <.0001 |
| Smoker | 40 (12) | 499 (15.4) | .11 |
| Dyslipidemia | 113 (34.0) | 1401 (43.2) | .0015 |
| Atrial fibrillation | 49 (14.7) | 460 (14.2) | .86 |
| Pain onset >6 h | 113 (34.0) | 955 (29.4) | .09 |
| Pain onset >12 h | 50 (15) | 294 (9) | .0006 |
| Anterior MI | 163 (49.1) | 1423 (43.9) | .08 |
| LVEF, % | 39 ±14 | 48.6 ±12 | <.0001 |
CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The data are expressed as No. (%) or mean±standard deviation.
Defined as a glomerular filtration rate <60mL/min and based on the first blood sample drawn after admission. Because renal function was unknown immediately before the episode, it remained unclear whether it arose from acute or pre-existing chronic renal insufficiency. Most likely it was a combination of both situations, which were both related to an adverse outcome.
Angiographic and procedural characteristics for the 2 groups are presented in Table 2. There were significant differences in many variables. Overall, patients in CS had more severe coronary artery disease. In these patients, there was a lower use of radial access, bivalirudin and drug-eluting stents and a higher use of IIb-IIIa inhibitors and intra-aortic balloon pumps. Factors independently associated with CS were LVEF (OR 0.93, 95%CI, 0.92-0.94; P <.0001) and renal failure (OR 2.38, 95%CI, 1.75-3.24; P <.0001).
Angiographic and Procedural Characteristics
| Cardiogenic shock | No cardiogenic shock | P | |
|---|---|---|---|
| n=332 | n=3244 | ||
| Radial access | 89 (26.8) | 1927 (59.4) | <.0001 |
| Baseline TIMI 0-I | 279 (84.0) | 2500 (77.1) | .005 |
| Diseased vessels | 2.05±0.85 | 1.82±0.8 | <.0001 |
| Lesions treated | 1.32±0.65 | 1.21±0.50 | .002 |
| Left main lesion | 33 (10) | 129 (4) | <.0001 |
| Left anterior descending lesion | 145 (43.6) | 1449 (44.6) | .77 |
| Multivessel disease | 216 (65.0) | 1766 (54.4) | .0003 |
| Bifurcation treated | 53 (16.0) | 309 (9.5) | .0003 |
| Bifurcation with 2 stents | 10 (3.0) | 51 (1.6) | .10 |
| NC lesions treated in primary PCI procedures | 61 (18.3) | 414 (12.8) | .0064 |
| NC lesions treated in staged PCI procedures | 22 (6.6) | 394 (12.1) | .0038 |
| NC lesions left untreated | 121 (36.4) | 1143 (35.2) | .70 |
| Thrombus aspiration | 179 (53.9) | 1821 (56.1) | .45 |
| DES | 70 (21.0) | 882 (27.2) | .018 |
| Stent length | 21.3±7.0 | 21.3±7.2 | .9 |
| Stent diameter | 3.04±0.4 | 3.02±0.4 | .38 |
| IVUS | 5 (1.5) | 47 (1.4) | .92 |
| Final TIMI III | 219 (66.0) | 3076 (94.8) | <.0001 |
| IABP | 150 (45.2) | 64 (2.0) | <.0001 |
| Bivalirudin alone | 24 (7.2) | 392 (12.1) | .008 |
| Bivalirudin+IIb-IIIa inhibitors | 0 (0.0) | 7 (0.2) | .88 |
| UFH alone | 203 (61.1) | 2120 (65.4) | .12 |
| UFH+IIb-IIIa inhibitors | 105 (31.6) | 725 (22.3) | .0002 |
DES, drug-eluting stent; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound; NC, nonculprit; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; UFH, unfractionated heparin.
The data are expressed as No. (%) or mean±standard deviation.
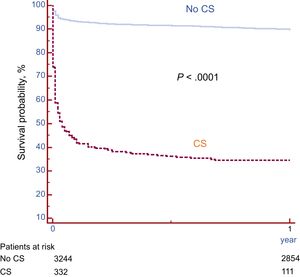
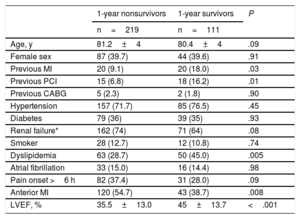
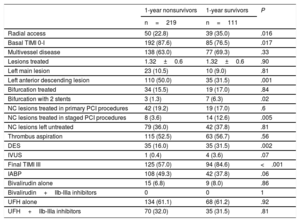
Survival free of death in patients with and without CS is shown in Figure 1. As expected, a large difference was observed, with much lower survival in patients with CS (34.1% vs 89.5% at 12 months; P <.0001). Among the 332 patients with CS, 180 (54.2%) died during hospital stay and 39 (11.7%) after discharge. Only 2 patients (0.6%) were lost to follow-up. The clinical characteristics according to 1-year survival status are described in Table 3. Survivors had more antecedents of ischemic heart disease, less anterior infarction, higher LVEF, and more dyslipidemia but showed trends for younger age, less renal failure, and shorter delay times. Analysis of angiographic and procedural variables showed that the use of radial access, drug-eluting stents and staged PCI procedures, as well as treatment of the anterior descending artery, were more frequent in survivors. In survivors, baseline flow was less frequently TIMI flow 0 to I and final TIMI III flow was more frequently achieved (Table 4).
Clinical Characteristics According to 1-year Survival
| 1-year nonsurvivors | 1-year survivors | P | |
|---|---|---|---|
| n=219 | n=111 | ||
| Age, y | 81.2±4 | 80.4±4 | .09 |
| Female sex | 87 (39.7) | 44 (39.6) | .91 |
| Previous MI | 20 (9.1) | 20 (18.0) | .03 |
| Previous PCI | 15 (6.8) | 18 (16.2) | .01 |
| Previous CABG | 5 (2.3) | 2 (1.8) | .90 |
| Hypertension | 157 (71.7) | 85 (76.5) | .45 |
| Diabetes | 79 (36) | 39 (35) | .93 |
| Renal failure* | 162 (74) | 71 (64) | .08 |
| Smoker | 28 (12.7) | 12 (10.8) | .74 |
| Dyslipidemia | 63 (28.7) | 50 (45.0) | .005 |
| Atrial fibrillation | 33 (15.0) | 16 (14.4) | .98 |
| Pain onset >6 h | 82 (37.4) | 31 (28.0) | .09 |
| Anterior MI | 120 (54.7) | 43 (38.7) | .008 |
| LVEF, % | 35.5±13.0 | 45±13.7 | <.001 |
CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The data are expressed as No. (%) or mean±standard deviation.
Two patients lost to follow-up were not included.
Angiographic and Procedural Characteristics According to 1-year Survival
| 1-year nonsurvivors | 1-year survivors | P | |
|---|---|---|---|
| n=219 | n=111 | ||
| Radial access | 50 (22.8) | 39 (35.0) | .016 |
| Basal TIMI 0-I | 192 (87.6) | 85 (76.5) | .017 |
| Multivessel disease | 138 (63.0) | 77 (69.3) | .33 |
| Lesions treated | 1.32±0.6 | 1.32±0.6 | .90 |
| Left main lesion | 23 (10.5) | 10 (9.0) | .81 |
| Left anterior descending lesion | 110 (50.0) | 35 (31.5) | .001 |
| Bifurcation treated | 34 (15.5) | 19 (17.0) | .84 |
| Bifurcation with 2 stents | 3 (1.3) | 7 (6.3) | .02 |
| NC lesions treated in primary PCI procedures | 42 (19.2) | 19 (17.0) | .6 |
| NC lesions treated in staged PCI procedures | 8 (3.6) | 14 (12.6) | .005 |
| NC lesions left untreated | 79 (36.0) | 42 (37.8) | .81 |
| Thrombus aspiration | 115 (52.5) | 63 (56.7) | .56 |
| DES | 35 (16.0) | 35 (31.5) | .002 |
| IVUS | 1 (0.4) | 4 (3.6) | .07 |
| Final TIMI III | 125 (57.0) | 94 (84.6) | <.001 |
| IABP | 108 (49.3) | 42 (37.8) | .06 |
| Bivalirudin alone | 15 (6.8) | 9 (8.0) | .86 |
| Bivalirudin+IIb-IIIa inhibitors | 0 | 0 | 1 |
| UFH alone | 134 (61.1) | 68 (61.2) | .92 |
| UFH+IIb-IIIa inhibitors | 70 (32.0) | 35 (31.5) | .81 |
DES, drug-eluting stent; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasound; NC, nonculprit; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction; UFH, unfractionated heparin.
The data are expressed as No. (%) or mean±standard deviation.
Two patients lost to follow-up were not included.
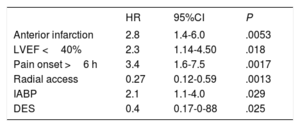
Independent predictors of 1-year mortality are presented in Table 5. Baseline and preprocedural variables identified as predictors were anterior infarction, LVEF <40%, and delay time from pain onset to PCI >6hours. Procedural factors independently associated with prognosis were the use of radial access, intra-aortic balloon pumps, and drug-eluting stents. Center allocation was not predictive.
Independent Predictors for 1-year Mortality
| HR | 95%CI | P | |
|---|---|---|---|
| Anterior infarction | 2.8 | 1.4-6.0 | .0053 |
| LVEF <40% | 2.3 | 1.14-4.50 | .018 |
| Pain onset >6 h | 3.4 | 1.6-7.5 | .0017 |
| Radial access | 0.27 | 0.12-0.59 | .0013 |
| IABP | 2.1 | 1.1-4.0 | .029 |
| DES | 0.4 | 0.17-0-88 | .025 |
95%CI, 95% confidence interval; DES, drug-eluting stent; HR, hazard ratio; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction.
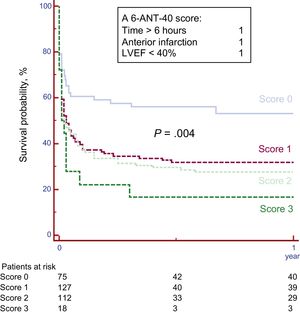
Based on these findings, a preprocedural predictive score was created including the 3 predictors, anterior location, LVEF <40%, and delay time >6hours. Patient survival according to the “6-ANT-40” score is shown in Figure 2. Outcomes differed significantly across the subgroups. Survival at 1 year was 54.5% for patients with score 0, 32.3% for score 1, 27.4% for score 2, and 17% for score 3 (P=.004, c-statistic 0.70).
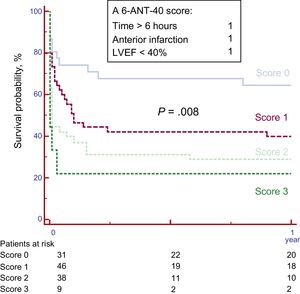
The validation cohort comprised 124 patients. Baseline characteristics are listed in Table 1 and . Overall 1-year survival for this cohort was 40% with 0.8% lost to follow-up. The survival curves according to the score values in the validation cohort are shown in Figure 3. One-year survival significantly differed across the score-based groups (64.5% for patients with score 0, 40.0% for score 1, 28.9% for score 2, and 22.2% for score 3 (P=.008, c-statistic 0.68).
DISCUSSIONThe main findings of this study can be summarize as follows: a) the incidence of CS in patients older than 75 years undergoing primary PCI was 9.3%; b) LVEF and renal failure were independent predictors of CS; c) CS was associated with very high mortality, which is concentrated in the hospitalization period; d) a preprocedural score based on 3 simple clinical variables (anterior location, LVEF <40%, and delay time >6 hours) may be used to estimate survival after PCI and to guide decision-making.
In this registry, mortality was high, especially during hospital admission, when slightly more than half the patients died. These results are in the range of the fatality rates previously reported in other registries.6,8–11 Of interest, among discharged patients, 1-year mortality was around 10%, which is not too high. Among the identified independent predictors of mortality, 3 were baseline preprocedural variables and the other 3 corresponded to procedural factors. Regarding the latter, there is a high probability of bias. Higher-risk patients (with more profound CS) could have been more frequently treated with intra-aortic balloon pumps (IAPB) whereas lower-risk patients (with less profound CS) could have had more radial access and implantation of drug-eluting stents. The use of IAPB showed no benefit in the subgroup of elderly patients in the IABP-SHOCK II trial.17 Drug-eluting stents have not been associated with improved survival in patients with CS.18 Concerning radial access, the reduction in mortality found in trials conducted in STEMI patients could suggest a certain protective role of this vascular access for patients with CS.19,20
Regarding revascularization strategies for multivessel disease, there were more staged procedures in the survivor group. Nonetheless, this difference was due to a not inconsiderable selection bias, since only patients surviving the critical first 3 to 4 days of MI could undergo a staged procedure.
The combination of the 3 clinical predictive factors (anterior infarction, LVEF <40%, and delay time >6 hours) into a score, assigning 1 point for each factor present, allowed patient stratification according to prognosis. This score was validated in an independent cohort. The score based on preprocedural clinical variables could be helpful to guide decisions in these highly complex situations.
Only a few studies have proposed a risk score for patients in CS undergoing contemporary primary PCI in STEMI and none of them was designed for elderly patients. The score proposed by García-Álvarez et al., 21 was based on 74 patients, which is a small sample for that purpose. More recently, 2 scores have been published for predicting mortality after primary PCI in the overall population with CS.22,23 The first was derived from 388 patients treated between 1995 and 2013, included 3 variables (age >75 years, cardiac arrest at presentation, and primary PCI failure), and showed a c-statistic of 0.66 for 2-year cardiac mortality.22 The second was derived from the IABP-SHOCK trial and was validated in independent registries.23 This score for prediction of 30-day mortality includes 6 variables: age >73 years, history of stroke, glucose >191 mg/dL, creatinine >1.5 mg/dL, lactate >5 mmoL/L, and TIMI flow <3 after PCI. The c-statistic was 0.79 and 0.73 in the derivation and validation cohorts, respectively. Nonetheless, these scores were derived from the overall population and so their value in the elderly population is limited. Both scores include the results of primary PCI and therefore they cannot be used to properly estimate outcomes and guide decision-making before primary PCI. The first score was obtained from a cohort of patients treated from 1995 to 2013, and therefore partially included noncontemporary PCI. Furthermore, it was not validated in another cohort. In addition, the antecedent of resuscitated out-of-hospital cardiac arrest is much more uncommon in patients older than 75 years and is therefore much less useful.22 In addition, the second score requires 3 laboratory determinations at admission.23 The serum concentration cutoff levels, especially for creatinine, may not be adequate for prognostic discrimination in the elderly and in general these cutoff values are highly vulnerable to differences in the population, the time of sampling, and laboratory methods. All these factors hamper the widespread implementation of this score in practice.
In contrast, our score was specifically derived and validated in an elderly population undergoing contemporary primary PCI and is based on 3 quite simple preprocedural clinical variables. Given these unique characteristics, the 6-ANT-40 score may be used in the decision-making process before primary PCI, which is particularly relevant in the setting of very elderly patients with CS.
We strongly believe that elderly patients with STEMI and CS should not be excluded from the potential benefits of primary PCI, but we should acknowledge that they obtain limited benefits from this procedure. Moreover, in some patients, PCI and subsequent further interventions could turn into an aggressive therapeutic approach leading to a kind of dysthanasia. Our study provides a predictive score based on simple clinical variables that may help to guide decision-making in this complex scenario.
LimitationsThis is a retrospective registry and, although consecutive inclusion was strongly encouraged, it cannot be fully guaranteed. Elderly patients with CS complicating STEMI but not considered for PCI (mostly deemed to have a very dismal short-term prognosis) were not included in this registry. Nevertheless, the results are applicable to the growing population of elderly patients with CS who are generally selected for PCI. The application of diagnostic criteria for CS was uniform but final allocation was based on the investigator's decision and some heterogeneity could exist. The identification of a prognostic value for procedural factors seems to be somewhat affected by a selection bias. Thus, these associations should be interpreted with caution and only as hypothesis-generating.
CONCLUSIONSThe incidence of CS in patients older than 75 years undergoing primary PCI was 9.3%. CS was associated with very high mortality concentrated in the hospitalization period. A preprocedural score based on 3 simple clinical variables (anterior location, LVEF <40%, and delay time >6 hours) may be used to estimate survival after PCI and to guide pre- and postprocedural decision-making.
- –
Because of population aging in western countries, there is an increase in the proportion of patients admitted with a diagnosis of STEMI.
- –
CS is more frequent in this population and dramatically increases the short-term mortality of these patients, even when treated with primary angioplasty.
- –
There is a scarcity of information on clinical profiles, outcomes, and predictors of both short- and long-term mortality in elderly patients with CS complicating STEMI after primary angioplasty.
- –
This study comprises a large series of patients older than 75 years with CS complicating STEMI and undergoing primary angioplasty, which allowed complete characterization along with the identification of predictors of CS, clinical outcomes, and prognostic variables.
- –
A prognostic score based on baseline clinical variables has been developed and validated for this specific population. This score predicts their clinical outcomes fairly well after an eventual primary angioplasty and therefore it could be implemented in the preinterventional decision making process.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2018.09.001.