Technological progress in medicine is constantly garnering pace, requiring that physicians constantly update their knowledge. The new wave of technologies breaking through into clinical practice includes the following: a) mHealth, which allows constant monitoring of biological parameters, anytime, anyplace, of hundreds of patients at the same time; b) artificial intelligence, which, powered by new deep learning techniques, are starting to beat human experts at their own game: diagnosis by imaging or electrocardiography; c) 3-dimensional printing, which may lead to patient-specific prostheses; d) systems medicine, which has arisen from big data, and which will open the way to personalized medicine by bringing together genetic, epigenetic, environmental, clinical and social data into complex integral mathematical models to design highly personalized therapies. This state-of-the-art review aims to summarize in a single document the most recent and most important technological trends that are being applied to cardiology, and to provide an overall view that will allow readers to discern at a glance the direction of cardiology in the next few years.
Keywords
In the second half of the 20th century, the emergence of information and communications technology and computer science ushered in a revolution that continues to provide society with technological advances. These advances have also greatly benefited the field of cardiology.
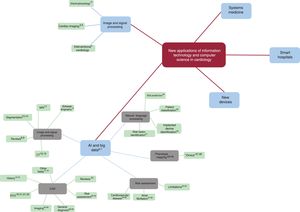
This revolution is far from over, and it continuously produces innovations that make it vitally important to keep up to date because of their disruptive potential. This article reviews the current panorama regarding the most recent technological advances in computer science and information technology that are affecting the world of cardiology. We have collected the most recent and significant examples of these advances and have created a concept map that classifies them into 5 main categories (figure 1): a) signal and image processing; b) artificial intelligence (AI) and big data; c) new devices; d) smart hospitals; and e) systems medicine.
SIGNAL AND IMAGE PROCESSINGAdvances in computer science are leading to continual improvements in medical imaging sensors and instruments. These devices are of immense usefulness, despite their being the most continuous and least disruptive example of technological progress. Manufacturers incorporate many of these advances into patent-protected devices. The literature is also replete with applications in several areas (figure 2):
- •
Electrophysiology: new algorithms improve implanted devices: for example, by reducing inappropriate shocks1 or by eliminating artifacts produced by cardiopulmonary resuscitation movements in the electrocardiogram (ECG) signals from automatic defibrillators.2
- •
Noninvasive imaging: new techniques allow measurements to be conducted automatically or semiautomatically rather than by hand, thereby significantly reducing the amount of time needed by a human operator. For example, the parameters needed to plan the percutaneous implantation of a prosthesis3 or to segment the left atrial appendage4 can be identified up to 100 times faster under computed tomography (CT).
- •
Interventional cardiology: an example is provided by calculation of the quantitative flow ratio, which is a functional estimation of coronary lesions obtained from a 3-dimensional reconstruction based on angiography without the need for additional measurement procedures.5
Concept map of advances in image and signal processing, artificial intelligence (AI), and big data. The literature references to each concept are indicated with superscript numbers. CAD, computer-aided diagnosis; CT, computed tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging.
Artificial Intelligence (AI) can be a vague concept, but Kaplan's definition6 is a good starting point: “a system's ability to correctly interpret external data, to learn from such data, and to use those learnings to achieve specific goals and tasks through flexible adaptation“.
Furthermore, there are very many AI-related terms that are often misleading. These include the following:”machine learning“, which is that part of AI used to analyze datasets to draw conclusions;”big data“, which, strictly speaking, only refers to the management and exploitation of large volumes of data, but which is a task for which AI techniques are often currently used; or”deep learning“, which is a subset of machine learning techniques that focus on learning from large volumes of data.
AI typically requires more system resources than other computing techniques. However, it obtains very good results in many fields and very powerful computers are currently available that can handle these additional processing loads.
The application of AI to cardiology is already a fact, as described by Dorado-Díez et al.7 in their review that classified different uses by areas of cardiology. In the present article, we group these uses according to the functions conducted by AI in order to offer an overview of its potential contribution regardless of specialty (figure 2).
Big data and imaging techniques in cardiologyAI techniques are particularly well suited to cardiac imaging because of the large volume of structured data that is stored in the images. There are many illustrative examples,8,9 of which we highlight the following: an algorithm to measure epicardial adipose tissue on CT10 reduces analysis time from 10minutes to 26seconds, and provides high correlations with human experts (R=0.92); a system to calculate the volume of myocardial fibrosis on magnetic resonance imaging (MRI) at 0.15 s per Image11 showed a high correlation with human experts (R=0.88); and an algorithm for automatic calcium scoring in CT has been applied to 7240 heterogeneous studies.12
The challenge of the automated segmentation of cardiac structures has attracted strong interest. Correlations between some MRI algorithms and human experts have reached 0.98 for some specific tasks,13 and fully automated segmentation in MRI is a real possibility in the near future. Echocardiography is a more complex challenge, but it is also producing spectacular results, such as the 3-dimensional segmentation system of the left ventricle proposed by Dong et al.14
Computer-aided diagnosisThe most natural application of AI is likely to be computer-aided diagnosis (CAD), which is understood as the identification of diseases with minimal supervision by human physicians. The first forays into CAD systems were attempted a few decades ago. They were deterministic systems that applied expert knowledge (ie, rules written by physicians), and until the 1990s the general consensus was that these automated systems were expensive, complex, and produced poor results.15,16
In contrast to this approach, modern systems use machine learning: the system is trained by presenting it with a series of cases with known diagnoses, so that the algorithm learns the characteristics of different groups without human operators necessarily having to know them. Once trained, the system can search for the learned parameters in cases with an unknown diagnosis. The major advantage of AI is that it can identify complex mathematical relationships between parameters, which otherwise would be very difficult for humans to identify with the naked eye.
Current CAD systems are already in a position to compete with human physicians. CAD systems have already been successful in various areas of medicine: for example, there are some CAD systems that are better than expert dermatologists at identifying tumors in photographs of skin lesions17 or better than expert ophthalmologists at detecting urgent retinal conditions.18
In the field of cardiology, computational techniques have been successfully applied to processing ECG signals. For example, the arrival of AI has led to the development of a spectacular system that can detect patients with atrial fibrillation (AF) by analyzing just 10seconds of a standard ECG during sinus rhythm.19 Furthermore, AI-based solutions have improved the performance of current tools20: for example, in 1 study, ambulatory single-lead ECG monitoring devices identified 12 types of arrhythmias with 6% greater accuracy than a panel of 3 experts.21
In the field of image processing, algorithms have been developed that can achieve 93% accuracy in the diagnosis of acute myocardial infarction on CT,22 obtain results comparable to those of experts and better than those of interns when detecting regional wall motion abnormalities on ECG,23 or detect chronic myocardial infarction on MRI with a sensitivity and specificity of 90% and 99%, respectively.24 We highlight the very thorough work of Zhang et al.,25 who obtained excellent results in the diagnosis of hypertrophic cardiomyopathy, cardiac amyloidosis, and pulmonary arterial hypertension. Litjens et al.26 made an exhaustive review of more than 80 different techniques for automated cardiovascular image analysis.
Most CAD tools have the following characteristics:
- •
The AI performs very specific tasks using highly specific data, and it is rare to find tools that integrate several different data sources or that are able to conduct general diagnoses. Some18 AIs can surpass experts when interpreting a specific test, but experts perform better than AIs when other general data are included, such as the patient's medical history.
- •
It is clear that there is a very long way to go before AIs can replace human physicians. However, there is room for AI tools in current clinical practice. These include the following:
- a.
Assistance tools that save time when conducting specific simple and repetitive tasks.
- b.
Triage tools that provide an initial classification in the setting of primary care and reduce specialist workload.
- c.
New improved diagnostic tools: for example, algorithms to process the ECG to improve the use of N-terminal prohormone of brain natriuretic propeptide (NT-proBNP) in the diagnosis of ventricular dysfunction.27
The Watson system (IBM, United States) is notable example of AI causing controversy relating to general diagnosis. This system has been applied to cancer diagnosis and treatment recommendations. Although studies have shown promising results with up to 93% consensus with experts,28 the system has also received substantial criticism from both inside29 and outside30 the scientific community. The accuracy of these figures has been challenged, including internal reports from IBM on cases in which the AI recommended inappropriate or dangerous treatments.
- •
At best, AI is only as good as the data it learns from. This means that any bias in the group of training cases will be reproduced in the final result.
- •
For this reason, great care must be taken when interpreting the accuracy of the results reported in the literature and equal care taken when examining the validation data. For example, very high accuracy may be distorted by selection bias caused by including cases with low diagnostic difficulty in the validation process.
In a meta-analysis, Liu et al.31 compared 82 articles published up to 2019 which addressed human experts vs CAD imaging systems. They suggested that although the current accuracy of automated diagnostic systems may be equivalent to that of health care professionals, caution is still required regarding validation methods and the data used.
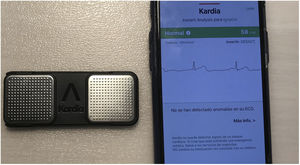
Finally, it should be noted that these AI applications are not limited to the research field, but are making their way into routine clinical practice. By 2019, the Food and Drug Administration had approved over 30 AI-based applications and systems for clinical use.32 Without doubt, one of the most popular is the KardiaMobile device (AliveCor, United States), which was approved by this agency for the detection of AF33 (figure 3).
Epidemiology and risk assessmentRisk assessment is a common practice that establishes patient prognosis and identifies the possible consequences of various treatment options. Traditionally, risk scores have been calculated using statistical tools. In this field, the application of AI follows a similar method34 in that a mathematical model is constructed in which input variables are related to the output variable to be predicted. However, AI has a fundamental advantage: more complex relationships can be detected by AI than by commonly used statistical tools, thus leading to more accurate predictions.
The literature has already presented solid evidence in favor of these AI algorithms. For example, in the setting of coronary heart disease, it has been shown that some risk scores created by AI algorithms provide greater prognostic accuracy (up to 25% improvement) than current statistical methods.35,36 Currently, there is no conclusive AI-based risk score for AF. Given the relevance of AF, there is a need for more studies on this issue,37 such as those already successfully conducted in small cohorts.38,39
In light of these results, the following conclusions can be summarized:
- •
AI has enormous potential to predict risk and it has been shown that it can strongly outperform traditional methods.
- •
The main disadvantage of AI-generated models is that they are not directly interpretable, unlike mathematical/statistical models.
- •
However, like statistical models, AI models are only as good as the data used to construct them and can incorporate any existing bias.
- •
The greater complexity of AI means that it typically requires more data to obtain good results. AI can identify information that is missed by other methods, but it cannot “work magic” and extract information that is not in the dataset.40 Therefore, if the dataset is too simple, only marginal improvements can be obtained with AI over statistical approaches.41 Moreover, as shown by Hernandez-Suarez et al.,42 AI can be less accurate in the prediction of mortality after transcatheter aortic valve replacement.
Phenotype mapping is one of the most disruptive applications of AI. When studying a disease, the patients’ characteristics are usually analyzed in order to classify them into different phenotypes corresponding to subdivisions or stages. Subsequently, each phenotype is studied individually. AI provides highly detailed and accurate phenotyping and can take into account parameters that no human researcher would be able to correlate. The price is having to accept that a calculator that performs difficult-to-understand operations will determine patient classifications.
The foremost example of this approach is provided in the study by Shah et al.43 Using AI techniques, these authors were able to find 3 phenotypes clearly differentiated by their prognosis and mortality rates for heart failure with preserved ejection fraction, which is a heterogeneous and elusive entity. This result represents a giant step forward in patient stratification and in establishing optimal treatments for each group.
This approach is particularly useful in the field of imaging. The number of imaginable radiological parameters is very large. Several algorithms are able to extract their own parameters from image analysis to subsequently identify groups of patients with similar parameters and better predict their progress. Examples include ventricular hypertrophy on CT,44 cardiovascular risk 5 years after a coronary CT scan,45 or pulmonary hypertension on MRI.46
The use of these analytic techniques is essential if cardiology is to harness the full power of the”omics” sciences (ie, genomics, proteomics, etc).47 Incorporating such data into the cardiovascular clinical process would usher in a new era of diagnostic and therapeutic accuracy and facilitate the identification of metabolic circuits and physiological causes.48
Natural language processingDespite the general trend toward building databases with more complex structures, much of the current information related to medical practice exists as free-form text in medical records. Fortunately, a field of AI known as natural language processing is capable of analyzing such information and extracting meaningful information.
Some AI applications can analyze medical histories to predict risk,49 while others extract and classify symptoms to categorize patients.50 In the field of cardiology, there are 2 interesting examples. The first is an algorithm that can surpass the predictive value of surveys and procedure codes in the identification of sudden cardiac death risk factors in hypertrophic heart disease.51 The second can detect the presence of MRI-incompatible implanted devices with an accuracy of 91%.52
Although a variety of real -world and successful clinical applications are currently available in other fields, natural language processing is still in the process of leaving the research environment.
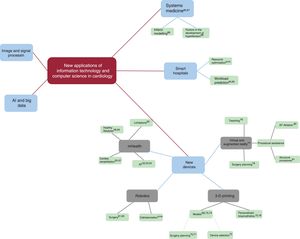
NEW DEVICESUp to this point, we have mainly presented the latest trends in software. In this section, we will present new developments in the field of hardware (figure 4).
Mobile health, or mHealth, is understood as the use of mobile devices and sensors to facilitate constant and ubiquitous health care. Smart devices provide first-line care 24hours a day, travel with patients wherever they go, and can conduct health monitoring tasks without human intervention. Smart devices include medical devices such as implantable defibrillators, but also include mobile phones, watches, clothing, and small sensors that can be carried at all times. Such devices have become very popular and can be used as follows:
- •
Continuously monitor and communicate with patients.
- •
Facilitate patients’ involvement in their own treatment.
- •
Monitor large populations.
For example, in the treatment of AF, some intelligent devices can detect episodes in at-risk patients with more than 90% accuracy.33,53 It has been shown that constant monitoring and communication through a mobile platform improves adherence, satisfaction with anticoagulants, and overall quality of life.54
There are also prototype applications on online platforms and smartphones for cardiac rehabilitation showing demonstrated improvements in functional capacity, oxygen consumption, and long-term exercise habits.55–57
Furthermore, mHealth is ideal for mass monitoring in the promotion of healthy lifestyles58 or for the detection of potentially dangerous arrhythmias.59 However, these large-scale applications probably require larger trials.
Many mHealth solutions are currently in the early stages of development, but despite their enormous potential, caution should still be exercised. Some trials have been unable to demonstrate the potential benefits of some platforms.60 However, some of these studies have marked methodological limitations, such as the use of short follow-up times or elderly populations.
RoboticsOne of the most disruptive trends is probably the robotization of cardiac intervention. The da Vinci robot (Abex, Spain) has been shown to have advantages over conventional techniques in a range of surgical settings, including cardiac surgery.61 In atrial septal defect closure, the results obtained with this device are similar to those of conventional techniques.62
Robotic-assisted percutaneous coronary intervention (PCI) has been shown to be noninferior to manual PCI,63 while providing some advantages.64 These include a lower incidence of dilatation beyond the edges of the stent,65 the ability to conduct interventions remotely,66 and exposure to up to 95% less radiation,67 which also reduces ergonomic issues caused by the weight of protective gear.
In the future, AI-integrated robots are expected to be able to offer advanced assistance during this procedure, early alerts on possible complications, and automated smart movements.68 Nevertheless, there are still substantial limitations, such as incompatibilities with cardiac catheterization equipment, hindrances to conducting complex interventions, and the need for manual arterial access.64
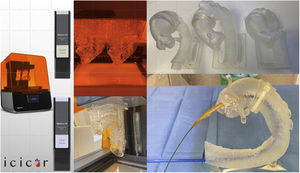
Three-dimensional printingThree-dimensional printed volumetric models based on imaging tests of real patients allow physicians to examine specific anatomies with their own hands (figure 5). This approach has multiple applications69:
- •
Preparation and planning of interventions.70,71
- •
Selection of optimal devices for specific anatomies.72
- •
Teaching.
- •
Testing of new devices.
Although some reports on this topic exist,73,74 studies with large numbers of patients are needed to determine the advantages of 3D printing as part of routine clinical practice In the future, 3D printed biomaterials could be used to produce personalized prostheses, such as coronary stents75 or perfusable and vascularized cardiac patches.76
Virtual and augmented realityVirtual reality (VR) immerses users in a 3-dimensional computer-generated world through headsets that are fitted with position and movement sensors. In contrast, augmented reality (AR) uses smartglasses with transparent screens on which additional information is projected in order to enrich the users’ vision of the real world. Although VR technology is already at an advanced stage, AR technology is still in an experimental stage. HoloLens smartglasses (Microsoft, United States) are the most popular device, but are expensive and relatively unknown (figure 6).
The application of both technologies to cardiology addresses 4 main aspects77:
- •
Simulation-based teaching.78
- •
Rehabilitation.
- •
Preprocedural planning.79
- •
Assisting interventional specialists during procedures by integrating 3D reconstructions of anatomical structures as guides to ablation80 or during structural procedures.81
Although these examples are anecdotal, these types of device are expected to become integrated into standard practice after their gradual launch onto the market. For example, in 2019, Philips introduced a system that integrates its Azurion angiography system with HoloLens smartglasses.
THE SMART HOSPITALA very complex challenge is managing the myriad processes in hospital health care. New technologies (figure 4) can also assist in this field by providing tools to automatically optimize the way work, resources, time, and personnel are organized and to facilitate the flow of information between all those involved in health care, including patients, hospital staff, physicians, and so on.
The implementation of these management tools is known as the smart hospital, which is a model for the future in which all processes are monitored and digitally optimized. The literature offers many examples, such as algorithms to organize operating rooms, wards, and consultations82,83 or to predict the duration of patient stays84 and daily admissions for cardiorespiratory problems.85
SYSTEMS MEDICINESystems medicine (figure 4) is the integration of genetic, molecular, cellular, and systemic mathematical models, as well as mathematical models of tissues and organs, into a single customizable virtual physiological human model. This model could be used to predict the specific effects of each intervention on a given patient, and identify possible crossinteractions in each part of the patient's body and each function. Such an approach would be the definitive form of personalized medicine and is considered by many to be the next great paradigm shift in medicine.86,87
Although this goal is still a distant one, the first steps have already been taken, such as the creation of personalized computational models of the heart to estimate the prognosis of patients who have experienced infarction,88 or to study the genetic, molecular, and environmental factors that lead to hypertension in individual patients.89
CONCLUSIONSWe have reviewed a group of technologies with enormous disruptive potential. Currently, the main highlight is the arrival of AI and mHealth: after many years of promises, data-based medicine is now a reality and is already changing the day-to-day practice of cardiologists. Although it is very unlikely that AI will suddenly replace cardiologists, it is highly probable that tools will appear in the short term to automate simple tasks. Specialists will be relieved of workloads related to repetitive and standard consultations and thus will be able to concentrate on more complex cases.
Some technologies appear to have matured, but have yet to find widespread clinical application. Good examples of the implementation of cheap fully-developed devices would be the mass use of mHealth devices to monitor the general population or the use of VR devices. However, these devices have not yet found their niche. Part of the problem is the lack of large-scale trials demonstrating quantitative benefits. Robotization and 3D printing appear to be stuck halfway. There are niches where they have demonstrated extraordinary usefulness, but they are still not in widespread use. Increased uptake will largely depend on the discovery of new applications and advantages that will make their use essential.
The real revolution will probably arrive with systems medicine. The integrated mathematical models of this approach will replace evidence-based statistics with personalized health care in which the cause of each symptom in each patient can be identified and the effect of each specific treatment can be accurately predicted. However, this technology is still in its infancy and it will be a long time before this goal can be achieved.
CONFLICTS OF INTERESTNone declared.
We would like to acknowledge Dr Alfredo Redondo, cardiologist at the Clinical Hospital of Valladolid and manager of the VAL 3D Lab, for providing some of the images that illustrate this article.