The ABSORB bioresorbable vascular scaffold (BVS), (Abbott Vascular, Santa Clara, California, United States) was granted a CE mark in 2011 and was met by the interventional community with high expectations and some skepticism. The expectations arose from the promise of full vascular restoration when the device disappears after a period of temporary scaffolding. Freedom from a metallic cage (ie, the cage left in place by metallic drug-eluting stents [DES]) is predicted to reestablish otherwise lost vessel features, including pulsatility, cyclical strain, and physiological shear stress.1 In contrast, skeptics pointed out the numerous technical challenges and limitations posed by these devices compared with current-generation DES.2 Indeed, the larger crossing profile, the bulky struts, and the propensity to fracture at high inflation pressures make BVS a “special case” in interventional laboratories. Will BVS ever become a workhorse device and replace DES from the shelves? In addition to technical ameliorations, 2 types of data are desirable to support this concept. First, the device needs to be proven at least noninferior to current-generation DES before bioresorption. In fact, the promise of a totally restored coronary artery in the long-term should not come at the price of increased failure in the short- and mid-term. Recent randomized studies with a 1-year follow-up in relatively uncomplicated patients and lesions are promising in this regard,3–6 and others are ongoing. Second and foremost, the device needs to be proven superior to DES after bioresorption. This objective is harder to investigate at present, because most of the BVS implanted worldwide in the last few years have not yet disappeared, which prevents us from meaningful considerations in the clinical setting. To look for long-term evidence of safety and efficacy, we need to go back to pivotal studies conducted in the late 2000s that included very few, selected patients but were conducted with foresight, using serial intravascular and noninvasive imaging, enabling investigation of at least some long-term surrogate endpoints. At present, these studies (ie, ABSORB cohorts A and B) represent the only crystal balls currently available to glimpse and predict the future of BVS.7–9
In ABSORB cohort A, 30 patients received the first iteration of the BVS device (BVS 1.0) on de novo lesions that were suitable for treatment with a single 3.0 x 12mm or 3.0 x 18mm scaffold. Angiographic endpoints were available at follow-up for 26 patients and intravascular-ultrasound endpoints for 24 patients. Optical coherence tomography was undertaken at baseline and follow-up in a subset of 13 patients. Finally, the transparency of BVS enabled acquisition of serial noninvasive multislice computed tomography imaging in 18 patients both at 18 months and 5 years, who represent the study population for the post hoc analysis by Campos et al10 published in Revista Española de Cardiología. In that study,10 the endpoint of interest was atheroma volume, an ultrasound-like metric presented here in 3 variants: a) percentage relative to the vessel volume; b) absolute value normalized to the mean segment length of the study population, and c) percentage and normalized serial change between the 2 study time points. These measures were compared between scaffolded segments in the treated vessel and 4 proximal segments in the 2 nontreated vessels. In the intervened segments (n = 18), there were no changes between 18 months and 5 years in percent atheroma volume and normalized total atheroma volume. In contrast, a significantly increased percentage and absolute atheroma volume were noted in nonintervened segments (n = 71 analyzable of 72 eligible). As a result, the change in percent atheroma volume at 5 years differed significantly between scaffolded regions and nonintervened segments (−1.2 ± 7.7% vs 2.7 ± 6.5%; P = .03), suggesting plaque stabilization or even regression in the former and plaque increase in the latter.
A strength of the study by Campos et al10 is the head-to-head comparison of scaffolded and unscaffolded segments in the same patient. Compared with traditional intergroup analyses, this study design ideally controls for the confounding effect that may arise from the unmatched prevalence of proatherogenic factors or the differential use of pharmacological strategies aimed at promoting plaque regression. However, the investigators’ decision to compare scaffolded segments with unscaffolded segments located in the proximal parts of the untreated vessels introduced a certain degree of imbalance that remained uncorrected, as reflected by the larger normalized vessel (217 vs 190mm3) and lumen (122 vs 96mm3) volumes in the group of nonintervened segments. Intravascular ultrasound studies have shown that plaque atheroma tends to develop more frequently in the proximal than in the mid and distal vessels, with a proximal-to-distal gradient.11,12 In the study by Campos et al,10 proximal coronary segments were more commonly analyzed in the control group; therefore, we cannot exclude the possibility that the lack of plaque progression observed in the BVS segments was more a reflection of lesion location than a true effect of the device. In addition, percentage regression is more likely to occur when the coronary burden is higher, as noted in the scaffolded segments included in this study compared with their counterpart (49% vs 45%). Finally, the ABSORB cohort A was a simple population with few atherogenic factors (ie, diabetes, prior myocardial infarction). These limitations challenge the authors’ conclusions, warranting further confirmatory studies.
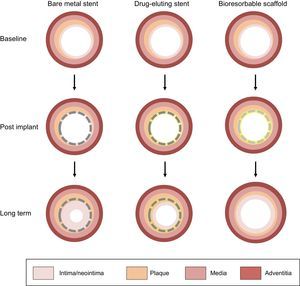
Plaque passivation has been described as one of the most interesting promises of BVS in the long-term.13 This effect has been attributed to the conjunction of symmetrical thick fibrous neointimal layering, lack of permanent vascular materials, and late lumen enlargement1 (Figure). The ability of neointimal development to shield the underlying plaque has also been described with bare metal stents.14 Unlike metallic stents, however, BVS may compensate the lumen narrowing induced by neointimal capping due to expansive remodeling.14,15 Campos et al10 now suggest another potential mechanism of plaque passivation with BVS, ie, local reduction of percentage plaque burden progression on top of pharmacological treatment. Indeed, everolimus has been advocated to decrease atherosclerotic plaque formation and inflammation in experimental models.16 However, the hypothesis of the drug being the main cause of the observed plaque regression contrasts with the notion that everolimus is mostly eluted during the first month in BVS, while plaque regression, if any, becomes apparent only after a couple of years. In addition, we cannot exclude the possibility that plaque regression is a “pseudo-phenomenon” linked to the disappearance of the polymeric struts followed by shrinking of connective tissue. Although the approach of studying the natural history of coronary artery disease with multislice computed tomography is pragmatic, optical coherence tomography and intravascular ultrasound remain the most accurate methods to discern the relative contribution of cap thickening, plaque regression, and vessel remodeling to the net passivating effect of BVS. Regardless of the underlying mechanism, the concept of plaque passivation with BVS, if proven true, would open the door to a dramatic paradigm shift in the percutaneous prevention of future coronary events. The idea of covering thin-cap fibroatheromas inducing plaque regression and formation of a thick shield of covering tissue is appealing. Importantly, this hypothesis currently lacks of an evidence base and is the subject of a specific proof-of-concept investigation (NCT02171065).
Natural history of contemporary coronary devices, with dramatized case examples. After bare metal stent implantation, neointimal tissue development contributes to shielding lipid tissues and covering metallic struts, but this occurs at the price of lumen narrowing. Drug-eluting stents counteract neointimal proliferation and restenosis, but the antiproliferative drug may induce delayed healing. In addition, the vessel loses its vasomotion properties due to permanent caging by metallic struts, which may act as triggers for potential late complications (ie, neoatherosclerosis). Bioresorbable scaffolds are suggested to induce neointimal tissue development covering the underlying tissue with no negative effects on lumen dimension, the latter being counterbalanced by plaque regression and expansive remodeling.
In conclusion, one may remain confused about the weight that can be attributed to post-hoc imaging studies from small first-in-man series such as the ABSORB A cohort. Patients and lesions treated in daily clinical practice are less selected, and even the marketed device is now different from that used at the time of the trial. Indeed, the ABSORB investigators should be applauded for their seminal and rigorous commitment to the understanding of the long-term properties of BVS. However, the remarkable amount of imaging data collected contrasts with the small and fragmented number of “cherry-picked” patients available at follow-up. In addition, the link between clinical outcomes and surrogate intravascular imaging endpoints of BVS is yet to be demonstrated. Will all the putative and established late effects of BVS translate into fewer ischemic events? This is the answer that skeptical interventionalists are looking for, and whether the promises of BVS will come true is the great unknown of the years ahead. Vascular restoration therapy is a promised land where everything now looks idyllic. Long-term follow-up of large randomized studies vs best-in-class DES will determine whether the daydream continue or ends abruptly.
CONFLICTS OF INTERESTD. Capodanno received speaker's honoraria from Abbott Vascular.