Keywords
INTRODUCTION
Cardiac resynchronization therapy (CRT) with biventricular pacing (BIV) is a good alternative for symptomatic treatment of patients with advanced heart failure and atrioventricular/intraventricular conduction disorders.1,2 First degree atrioventricular block and an associated QRS>120 ms, specifically by left bundle-branch block, produces an atrioventricular, interventricular and intraventricular dysynchrony that leads to an ineffective atrial filling contribution, produces early mitral regurgitation, shortens diastole and isovolumic contraction, and discordinates ventricular systole reducing its efficiency.3-5 Atrio-biventricular pacing reduces these alterations to a minimum, as it reduces atrial, atrioventricular and ventricular conduction delays.6-8
The principal studies confirm this technique improves functional capacity, quality of life and acute hemodynamic parameters, although there is still no conclusive data on its effects on survival.9-13
The initial procedures of left ventricular electrode implantation using thoracotomy have changed to epicardial transvenous access through the coronary sinus tributary veins, with electrodes and materials designed for this purpose. The current technique is minimally invasive but still challenging, and comprises aspects of conventional anti-bradycardia pacing, electrophysiology and hemodynamics, as the most commonly accepted strategy is a multidisciplinary approach.14
In this article, we review the technical practice and clinical results of an initial series obtained in a electrophysiology laboratory with conventional cardiac pacing expertise.
MATERIAL AND METHODS
Twenty-two consecutive patients with functional class III or IV congestive heart failure, left ventricular dysfunction (EF<0.35) and intraventricular conduction disorder (QRS>120 ms), underwent CRT therapy between October, 2000, and May, 2002. Four received an implantable defibrillator with BIV pacing capacity (Guidant H135 or 1823), and 18 received an anti-bradycardia device Guidant 1241 (17 patients), or Pacesetter 5510 (1 patient). Guidant LV electrode systems 4513 were implanted in 21 patients, and St. Jude Medical 1055K in one patient.
The baseline study included a physical examination, a 12-lead electrocardiogram and a color echo-Doppler study. Eight patients underwent previous electrophysiologic examination for different reasons.
Table 1 summarizes clinical baseline characteristics and implantation indications.
Pacemaker implantation
All the implantations were performed in an electrophysiology ward equipped with a Hicor type angiography digital system (Siemens). The procedure was performed using local anesthesia, or spontaneously ventilated general sedation during induced ventricular fibrillation in the case of defibrillators. Electrodes were generally implanted first in the right atrium (atrial appendix, active fixation) and then in the right ventricle (apex, passive fixation), using the common techniques previously described.15 Specifically, each electrode had one venous access. For patients having their previous anti-bradycardia pacemaker replaced, radioscopic monitorization was used during puncture to prevent damages to existing electrodes. After inspection of right electrodes, the coronary sinus ostium was cannulated using the guiding catheter. This was aided by angiography and intracavitary electrocardiograms registered in a Bard Labsystem electrophysiology recording system using 6F Cordis steerable electrodes. The guiding catheter was positioned 5 cm approximately from the coronary sinus ostium, advancing it generally through the electrocatheter. Coronary sinus venographies were obtained in anteroposterior projection, and occasionally, left-anterior oblique projection, by means of manual injections of contrast agent through a Berman type balloon catheter. Electrodes were implanted preferably in LV lateral, posterolateral or anterolateral regions, aiming to obtain a <2 V pacing threshold in 0.5 ms, as well as a wide separation between electrodes of both ventricles. Radiologic controls and threshold measurements were performed after implantation and before discharge for detecting possible left ventricular electrode dislocation and assuring LV capture. The optimal atrioventricular interval pre-discharge schedule was realized using echocardiographic control and the techniques previously described in all cases.16
Follow-up
Three months after implantation, patients underwent a clinical evaluation, a pacemaker control with pacing threshold evaluation, and echocardiographic follow-up with LV and left atrium EF calculation by automatic boundary detection.
Aerobic functional capacity acute testing
Three months after implantation, each patient underwent two aerobic functional capacity tests in less than one week with spirated gases metabolic analysis; one of the tests was performed during BIV pacing, and the other test with the pacemaker disconnected, or with right ventricular pacing if patients were pacemaker-dependent. Each patient took the aerobic functional capacity acute tests in randomized order. Pre-implantation aerobic functional capacity tests were not performed.
Statistical analysis
Numerical results are expressed as mean (standard deviation) and were analyzed with a SPSS 10.0 database. Variables differences before and after CRT were evaluated using a non-parametric test for related samples (Wilcoxon), and the t test for non-related samples when variables of different groups were compared. The differences were considered significant when P<.05.
RESULTS
Pacemaker implantation
The left ventricular electrode was initially implanted with success in 21 of the 22 patients (95%). In the unsuccessful case, advancing the guiding catheter in the coronary sinus resulted impossible despite catheterization with the electrophysiology catheter. Electrode final position was in the posterolateral region in 8 patients, in the lateral region in another 8 (Figure 1), in anterolateral position in 3 (Figure 2), and in anterior position in 2. The time intervals needed for the procedural stages are summarized in Table 2. No significant differences were found (P=.31) in time used for implantation (skin-to-skin) between the first 17 patients and the last 5 patients. LV unipolar pacing threshold was 1.53 (1.04) V in 0.5 ms, with a 848 (207) ‡ pacing impedance and a 10.5 (5.1) mV R-wave. As regards illness, indication of ischemic heart disease was associated to worse pacing thresholds (1.69 V compared to 0.76 V; P=.01) and longer mean total implantation times (129 min compared to 87 min; P=.025). Mean hospital stay after implantation was 2.8 (0.9) days. The only complications to mention were one pneumothorax with total collapse of the left lung that required drainage and resolved without sequels, and one thrombosis in the posterolateral branch where the electrode had been manipulated (discovered incidentally during a control venography), that evolved totally asymptomatic. Three patients (14%) suffered a LV electrode dislocation, with loss of left ventricular capture in one and an unacceptable increase in pacing threshold in the other two patients. All had the electrode repositioned, in two cases successfully, while the third case did no present unipolar pacing thresholds lower than 3.7 V without diaphragmatic stimulation. This made pseudobipolar mode chronic pacing impossible with our generator. At the end, 20 of the 22 patients (91%) were receiving left ventricular pacing at discharge.
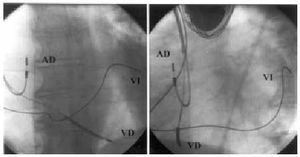
Fig. 1. Left ventricular electrode implanted in baseline lateral region (left, postero-anterior projection; right, left-anterior oblique projection). RA indicates right atrium; RV, right ventricle; LV, left ventricle.
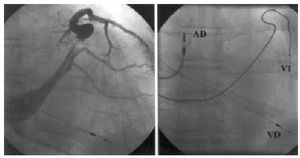
Fig. 2. Electrode implanted in mid-anterolateral region (left, venography in postero-anterior projection; right, in postero-anterior projection). Left-anterior oblique projection confirmed electrode anterolateral positioning. RA indicates right atrium; RV, right ventricle; LV, left ventricle.
Clinical follow-up and aerobic functional capacity acute testing
None of the 20 patients undergoing implantation successfully lost their left ventricular pacing during follow-up -- 200.6 (82) days -- and 18 were alive in May, 2002. One patient -- with extense anterior acute myocardial infarction in 1992, EF 0.15 and functional class III -- died suddenly while sleeping four months after pacemaker implantation. He underwent an electrophysiologic examination without induced sustained ventricular arrhythmias before implantation. This patient was considered non-respondent and had been included in the waiting list for cardiac transplantation. Another patient that showed functional class improvement died five months after defibrillator implantation, due to septic shock of nephrourinary origin.
Two patients did not undergo any echocardiographic examinations or functional capacity tests, as they were already followed-up in other centres, although their survival and persisting left ventricular pacing was checked after three months.
Chronic pacing thresholds (1.7 V), impedance (785 Ω) and R-wave voltage (10.5 mV) measured after three months did not show significant differences with respect to pre-discharge values. Clinical, electrocardiographic and echocardiographic characteristics before and after implantation are summarized in Table 3.
During the 3 months after implantation functional class improvements were noticeable (3.4 compared to 2.3; P=.039), and admissions due to heart failure were much fewer (3.12 vs 1.38; P=.001), as compared to 3 months before the procedure. In the follow-up echocardiographic examination, left ventricular EF was higher with pacemaker programmed in BIV pacing (0.29) than with right ventricular pacing or intrinsic rhythm (0.24; P=.042), and higher than pre-implantation EF (0.23; P=.038). Independent analysis of the 6 patients with their implanted antibradycardia pacemaker replaced showed similar results, such as significant functional class improvements and fewer admissions due to heart failure.
The acute metabolic study performed three months after implantation showed a noticeably higher peak oxygen uptake (P=.028) in patients programmed in BIV pacing (14.89 mL/min/kg) than when they underwent the stress test with intrinsic rhythm or right ventricular pacing (12.65 mL/min/kg) (Figure 3).
Fig. 3. Peak oxygen uptake obtained in post-implantation acute testing with/without resynchronization. BIV indicates biventricular; AT, anaerobic threshold; UniV, right ventricular pacing or intrinsic rhythm; VO2, oxygen uptake.
Three patients of the entire group receiving BIV pacing (14%) did not show any clinical improvements. One died suddenly in the circumstances previously mentioned and one female patient underwent a cardiac transplantation 5 months after implantation.
DISCUSSION
Technological innovations have generalized the CRT technique by using minimally invasive procedures that allow to select of the LV region to be paced.14 Our implantation success rate is similar to other recent series that attained 84% to 92% left ventricular pacing.9,17 We have to consider that some procedures were very time-consuming; in fact, 40% of our cases showed a longer than 120 min duration with up to 116 min radioscopy intervals. To attain high success rates, we recommend using a radioscopy system with sufficient resolution for viewing 0.014" guides and integrated image storage and comparison functions, without time as a limiting factor. Also, coronary sinus catheterization can be facilitated by using conventional electrophysiology electrocatheters, or in extremely difficult cases, using steerable catheters and registering the intracavitary electrocardiogram. In this respect, we should remember that coronary sinus inaccessibility is the second most frequent cause of implantation failure in most series.18
In fact, failure to implant a left ventricular electrode that corresponded chronologically to one of the last patients of our series, was caused by the former. Another case in which sustained left ventricular pacing was not achieved showed a 3.7 V in 0.5 ms intraoperatory unipolar threshold, although capture was impossible with generator programmed in pseudobipolar mode at maximum energy output (7.5 V in 1.0 ms). This fact is already documented by other authors and could have been solved using a generator with a separate output for each ventricle,19 or perhaps, with bipolar electrodes. Implantation in ischemic heart disease patients needed more time and their left ventricular thresholds were higher. This reflects major technical difficulties probably caused by existing necrotic and unexcitable myocardial areas. In our opinion, 0.014" guiding coaxial electrode systems are extremely useful in these cases, as multiple myocardial areas can be mapped and cardiac venous system displacements are facilitated.20,21
Our study, due to its relatively small number of patients, its design aspects, and that some of the clinical variables analyzed are subjective (functional class, number of admissions), does not allow to ascertain that clinical benefits observed were due to therapy. Nevertheless, data obtained for our series is in agreement with results from most multicentric studies that demonstrate similar functional class and life quality improvements, and less admissions. Higher oxygen uptake associated to BIV pacing showing up in the post-implantation acute tests seems to be another improvement, with the necessary limitations of these acute tests. Three patients (14%) did not show any improvement. Existence of non-respondent patients with classical symptoms of atrioventricular and intraventricular dysynchrony makes it necessary to refine the criteria for predicting improvement. Also these criteria should be related to particular left ventricular pacing regions to maximize the hemodynamic benefit. Also, clarifying still not fully known issues such as the predictive factors of a favorable clinical response as well as the mechanisms influencing patient improvement, could be very useful to select patients taking advantage of the CRT techniques. Finally, therapy effects on survival is a major question to be clarified in the future by multicentric studies currently in execution.22
In our series, we included 6 advanced heart failure patients, already receiving RV apex chronic pacing due to complete atrioventricular block, for which there was no other therapeutical option available. Heart failure etiology was unknown in 2 cases and ischemic in the other 4 cases. Although RV apex chronic pacing generates an electrocardiographic pattern similar to left bundle-branch block, it does not necessarily have the same mechanical effect as the «natural» block. It has been demonstrated that intraventricular electromechanical delay, contraction and relaxation times, and ventricular wall dysynchronization magnitudes are not identical in both situations.23 It is also true that chronic pacing from the RV apex is associated with adverse hemodynamic effects.24-26 Clinical and experimental studies have shown histopathological changes that could cause ventricular remodeling at the long term.27-30 Such alterations might contribute to left ventricular function deterioration as «pacing induced cardiomyopathies» observed, not so rarely, in patients receiving RV apex chronic pacing.31 The improvements found in this subgroup agree with recently published data suggesting a benefit in updating these patients with left ventricular dysfunction and congestive heart failure receiving RV chronic pacing to BIV pacing.32,33 This possible new indication for CRT should be evaluated in the future in prospective studies designed for this purpose.
CONCLUSIONS
BIV pacing can be a valid therapeutic option for patients with heart failure and signs of atrioventricular and intraventricular dysynchrony. The implantation procedure, although difficult in some cases, is minimally invasive, safe and effective, with a higher than 90% success rate. Possible clinical benefits observed in acute tests after implantation, such as functional class improvement, number of admissions, and higher oxygen uptake associated with BIV pacing, need to be demonstrated in studies designed for this purpose. Randomized studies with long-term follow-ups are also necessary for evaluating the effect of this technique on survival, for refining the clinical improvement predictive criteria to select the patients, and for assessing its efficacy in other similar clinical situations, such as patients with left ventricular dysfunction receiving RV apex chronic pacing.
ACKNOWLEDGMENT
Our acknowledgment to Dr. Lluís Mont (Hospital Clínic, Barcelona) for his invaluable assistance in the first case of this series.
Correspondence: Dr. I. García-Bolao.
Departamento de Cardiología y Cirugía Cardiovascular.
Clínica Universitaria de Navarra.
Avda. Pío XII, s/n. 31008 Pamplona.
E-mail: igarciab@unav.es
Received 16 May 2002.
Accepted for publication 29 October 2002.