The impact of cancer on clinical outcomes in patients with atrial fibrillation (AF) is unclear. The aim of this study was to assess how cancer influences the prediction and risk of embolic and hemorrhagic events in patients with AF.
MethodsThe study population comprised 16 056 patients from a Spanish health area diagnosed with AF between 2014 and 2018. Of these, 1137 (7.1%) had a history of cancer. During a median follow-up of 4.9 years, we assessed the relationship between cancer and bleeding and embolic events by competing risk analysis, considering death as a competing risk.
ResultsNo association was detected between an increased risk of embolic events and cancer overall (sHR, 0.73; 95%CI, 0.41-1.26), active cancer, or any subgroup of cancer. However, cancer was associated with an increased risk of bleeding, although only in patients with active cancer (sHR, 1.42; 95%CI, 1.20-1.67) or prior radiotherapy (sHR, 1.40; 95%CI, 1.19-1.65). Both the CHA2DS2-VASc and HAS-BLED scores showed suboptimal performance to predict embolic and bleeding risk (c-statistic <0.50), respectively, in nonanticoagulated patients with active cancer. The ratio between the increase in bleeding and the decrease in embolisms with anticoagulation was similar in patients with and without cancer (5.6 vs 7.8; P <.001).
ConclusionsCancer was not associated with an increased risk of embolic events in AF patients, only with an increased risk of bleeding. However, active cancer worsened the ability of the CHA2DS2-VASc and HAS-BLED scores to predict embolic and bleeding events, respectively, in nonanticoagulated patients.
Keywords
Globally, 1 in 5 people develop cancer during their lifetime.1 Fortunately, recent advances in the detection, diagnosis, and treatment of many cancers have resulted in a progressive decline in mortality. Many oncological patients will live 5 or more years after their initial diagnosis, leading to an increased focus on addressing long-term medical and psychosocial needs. Based on data from the International Agency for Research on Cancer, in 2020 more than 50 million people worldwide were living with a cancer diagnosis made within the previous 5 years.1
Recent studies have shown that patients with a history of cancer have a 2-fold greater risk of developing cardiovascular disease than patients without cancer.2 Atrial fibrillation (AF) is a common cardiovascular comorbidity among patients with cancer,3 and the co-occurrence of these 2 disease states presents several clinical challenges. Prevalence rates of AF vary greatly from one study to the next, depending on whether the focus was on patients with active cancer, patients with a past history of cancer, or patients undergoing oncologic surgery. In the REGARDS study,3 patients with cancer were 20% more likely to have AF than those without cancer, reaching an AF prevalence of 15%. Since many cancers interact with the coagulation system, the use of anticoagulation therapy in AF patients with cancer can be difficult given unpredictable changes in thrombosis and bleeding risk.4,5 To help decision-making in this clinical dilemma, it would be very useful to provide information on how a previous diagnosis of cancer influences the risk of embolic and hemorrhagic events in AF patients. We therefore performed the present study, considering the influence of activity, timing, stage, and treatment of cancer on the embolic and hemorrhagic risk of AF patients.
METHODSStudy populationA retrospective registry-based cohort study including all consecutive patients (n=16 202) with a diagnosis of AF between January 2014 and January 2018 in the health area of Vigo (Galicia, Spain) was performed (CardioCHUVI-AF registry; ClinicalTrials.gov identifier: NCT04364516).6,7 Patients with missing baseline and follow-up data were excluded (n=146); therefore, the final cohort comprised 16 056 AF patients. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee (Autonomous Committee of Research Ethics of Galicia, code HAC-ACO-2018-01, registry 2018/258). Based on the retrospective and population-based nature of the study, the ethics committee did not consider informed consent necessary.
Patient and public involvementPatients and the public were not involved in any way in this study.
Cancer diagnosisCancer was defined as any malignancy diagnosed prior to the evaluation of the patient for AF, including blood and solid cancers. Myeloproliferative neoplasms, together with localized nonmelanoma skin cancer, benign tumors, and in situ precancerous lesions (eg, high-grade dysplasia), were not included in this analysis, given their anticipated low clinical relevance during the study horizon. Cancers were classified according to their activity, stage, and location. Active cancer was defined according to the last definition proposed by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, as cancer diagnosed within the 6 months before the medical evaluation for AF, cancer with local progression, metastasis, or lack of complete remission in the last 6 months, as well as patients receiving specific cancer therapy within the previous 6 months.8 Staging was based on the American Joint Committee on Cancer classification.9 Moreover, cancer types were classified by primary anatomic and systemic involvement, including only those locations in which the frequency of cancer was ≥ 40 patients (≥ 3.5% of all cancers in our study).
OutcomesThe primary outcomes were embolic and hemorrhagic events during follow-up. Embolic events were defined according to the endpoints for cardiovascular and stroke for clinical trials developed by the Standardized Data Collection for Cardiovascular Trials Initiative and the US Food and Drug Administration.10 Hemorrhagic events included major bleeding and clinically relevant nonmajor bleeding, defined according to the International Society on Thrombosis and Haemostasis.11,12
Statistical analysisWe used Fine-Gray proportional subdistribution hazards models to estimate the association of cancer with embolic and bleeding events. Death served as a competing risk. Effect estimates were reported as subdistribution HRs (sHRs) along with their 95% confidence intervals (95%CI). The incidence of both outcomes was estimated using weighted cumulative incidence curves. All analyses were adjusted for age, sex, CHA2DS2-VASc score, HAS-BLED score, and anticoagulation therapy. We complemented our analysis with a propensity score matching analysis. To match individuals, we used 1:1 nearest-neighbor matching, with a caliper of 0.1. A matched population of 2270 patients (1135 with cancer and 1135 without cancer) was obtained (). Following propensity score matching, the association between cancer and embolic or bleeding events was assessed with conventional competing risk models. The predictive performance of the included risk scores was assessed by their discrimination and calibration abilities. Discrimination was assessed by the concordance index (c-statistic), which reflects how well the risk score distinguishes between patients with and without the outcome of interest. For calibration, overall measures of global fit were examined, including the Brier score, which was calculated as the average squared deviation between predicted and observed risks. Statistical analyses were conducted using STATA and R software. A 2-sided P?value <.05 was considered statistically significant.
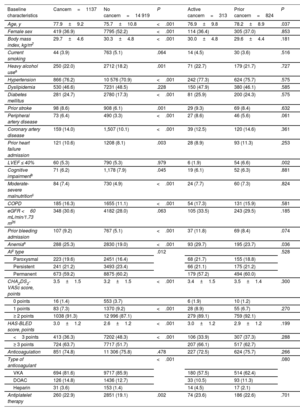
RESULTSStudy population and baseline characteristicsOf the 16 056 AF patients, a total of 1137 (7.1%) had a current or past diagnosis of cancer. Of all cancers, 313 (27.5%) were active (). A total of 419 (36.9%) were in advanced stages (III-IV), with metastasis in 86 patients (7.6%). The most common cancer locations were prostate (n=339; 29.8%), followed by colon-rectum (n=192; 16.9%), breast (n=155; 13.6%), kidney-bladder (n=93; 8.2%), blood (n=63; 5.5%), and lung (n=42; 3.7%). Radiotherapy was administered to 344 patients (30.3%), and 234 patients (20.6%) received chemotherapy (more information in ). The baseline characteristics of study population, comparing patients with and without cancer, are shown in table 1.
Baseline characteristics of the study population comparing patients with and without cancer
| Baseline characteristics | Cancern=1137 | No cancern=14 919 | P | Active cancern=313 | Prior cancern=824 | P |
|---|---|---|---|---|---|---|
| Age, y | 77.9±9.2 | 75.7±10.8 | <.001 | 76.9±9.8 | 78.2±8.9 | .037 |
| Female sex | 419 (36.9) | 7795 (52.2) | <.001 | 114 (36.4) | 305 (37.0) | .853 |
| Body mass index, kg/m2 | 29.7±4.6 | 30.3±4.8 | <.001 | 30.0±4.8 | 29.6±4.4 | .181 |
| Current smoking | 44 (3.9) | 763 (5.1) | .064 | 14 (4.5) | 30 (3.6) | .516 |
| Heavy alcohol usea | 250 (22.0) | 2712 (18.2) | .001 | 71 (22.7) | 179 (21.7) | .727 |
| Hypertension | 866 (76.2) | 10 576 (70.9) | <.001 | 242 (77.3) | 624 (75.7) | .575 |
| Dyslipidemia | 530 (46.6) | 7231 (48.5) | .228 | 150 (47.9) | 380 (46.1) | .585 |
| Diabetes mellitus | 281 (24.7) | 2780 (17.3) | <.001 | 81 (25.9) | 200 (24.3) | .575 |
| Prior stroke | 98 (8.6) | 908 (6.1) | .001 | 29 (9.3) | 69 (8.4) | .632 |
| Peripheral artery disease | 73 (6.4) | 490 (3.3) | <.001 | 27 (8.6) | 46 (5.6) | .061 |
| Coronary artery disease | 159 (14.0) | 1,507 (10.1) | <.001 | 39 (12.5) | 120 (14.6) | .361 |
| Prior heart failure admission | 121 (10.6) | 1208 (8.1) | .003 | 28 (8.9) | 93 (11.3) | .253 |
| LVEF ≤ 40% | 60 (5.3) | 790 (5.3) | .979 | 6 (1.9) | 54 (6.6) | .002 |
| Cognitive impairmentb | 71 (6.2) | 1,178 (7.9) | .045 | 19 (6.1) | 52 (6.3) | .881 |
| Moderate-severe malnutritionc | 84 (7.4) | 730 (4.9) | <.001 | 24 (7.7) | 60 (7.3) | .824 |
| COPD | 185 (16.3) | 1655 (11.1) | <.001 | 54 (17.3) | 131 (15.9) | .581 |
| eGFR <60 mL/min/1.73 m2d | 348 (30.6) | 4182 (28.0) | .063 | 105 (33.5) | 243 (29.5) | .185 |
| Prior bleeding admission | 107 (9.2) | 767 (5.1) | <.001 | 37 (11.8) | 69 (8.4) | .074 |
| Anemiae | 288 (25.3) | 2830 (19.0) | <.001 | 93 (29.7) | 195 (23.7) | .036 |
| AF type | .012 | .528 | ||||
| Paroxysmal | 223 (19.6) | 2451 (16.4) | 68 (21.7) | 155 (18.8) | ||
| Persistent | 241 (21.2) | 3493 (23.4) | 66 (21.1) | 175 (21.2) | ||
| Permanent | 673 (59.2) | 8875 (60.2) | 179 (57.2) | 494 (60.0) | ||
| CHA2DS2-VASc score, points | 3.5±1.5 | 3.2±1.5 | <.001 | 3.4±1.5 | 3.5±1.4 | .300 |
| 0 points | 16 (1.4) | 553 (3.7) | 6 (1.9) | 10 (1.2) | ||
| 1 points | 83 (7.3) | 1370 (9.2) | <.001 | 28 (8.9) | 55 (6.7) | .270 |
| ≥ 2 points | 1038 (91.3) | 12 996 (87.1) | 279 (89.1) | 759 (92.1) | ||
| HAS-BLED score, points | 3.0±1.2 | 2.6±1.2 | <.001 | 3.0±1.2 | 2.9±1.2 | .199 |
| <3 points | 413 (36.3) | 7202 (48.3) | <.001 | 106 (33.9) | 307 (37.3) | .288 |
| ≥ 3 points | 724 (63.7) | 7717 (51.7) | 207 (66.1) | 517 (62.7) | ||
| Anticoagulation | 851 (74.8) | 11 306 (75.8) | .478 | 227 (72.5) | 624 (75.7) | .266 |
| Type of anticoagulant | <.001 | .080 | ||||
| VKA | 694 (81.6) | 9717 (85.9) | 180 (57.5) | 514 (62.4) | ||
| DOAC | 126 (14.8) | 1436 (12.7) | 33 (10.5) | 93 (11.3) | ||
| Heparin | 31 (3.6) | 153 (1.4) | 14 (4.5) | 17 (2.1) | ||
| Antiplatelet therapy | 260 (22.9) | 2851 (19.1) | .002 | 74 (23.6) | 186 (22.6) | .701 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; eGFR: estimated glomerular filtrate rate; LVEF, left ventricular ejection fraction; VKA, vitamin K antagonist.
The data are expressed as No. (%) or mean±standard deviation.
Defined by National Institute on Alcohol Abuse and Alcoholism as consuming ≥ 4 alcoholic drinks on any day or ≥ 14 alcoholic drinks per week for men, or consuming ≥ 3 alcoholic drinks on any day or ≥ 7 alcoholic drinks per week for women, in the past month.
Equivalent to moderate-to-severe dementia, defined as cognitive decline between stages 5 and 7 of the Reisberg Global Deterioration Scale, which corresponds to stages of the Functional Assessment Staging scale ≥ 5.
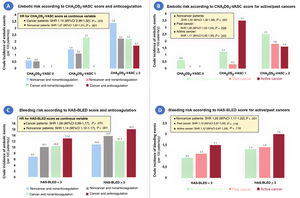
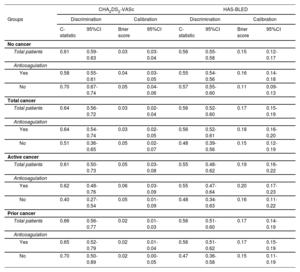
In contrast to what was observed in nononcological patients, a progressive increase in the rate of embolic and hemorrhagic events was not observed in patients with cancer as the CHA2DS2-VASc and HAS-BLED scores increased, respectively (). No patients with cancer and a CHA2DS2-VASc score of 0 had embolic events; however, with a CHA2DS2-VASc score=1, nonanticoagulated patients with active cancer had even more events than those with CHA2DS2-VASc score ≥ 2 (figure 1). In relation to the HAS-BLED score, patients with cancer and low bleeding risk (HAS-BLED score <3) had similar bleeding rates to patients without cancer and high bleeding risk (HAS-BLED score ≥ 3) (figure 1). The performance of the 2 risk scores is summarized in table 2. Discrimination of both CHA2DS2-VASc and HAS-BLED scores was optimal and similar (P> .05) between patient with past cancer and those without cancer but was poor in nonanticoagulated patients with active cancer (c-statistic 0.40 and 0.48 for CHA2DS2-VASc and HAS-BLED scores, respectively).
Embolic and bleeding risk according to the CHA2DS2-VASc and HAS-BLED scores, respectively, in atrial fibrillation patients with and without cancer, distinguishing between prior cancer and active cancer. Crude rates of embolic events in anticoagulated vs nonanticoagulated patients (A), and in those with past and active cancer (B). Crudes rates of bleeding events in anticoagulated vs nonanticoagulated patients (C), and in those with past and active cancer (D).
Performance of the CHA2DS2-VASc and HAS-BLED scores to predict embolic and bleeding events, respectively, in patients with and without cancer, according to anticoagulation status
| CHA2DS2-VASc | HAS-BLED | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Discrimination | Calibration | Discrimination | Calibration | ||||
| C-statistic | 95%CI | Brier score | 95%CI | C-statistic | 95%CI | Brier score | 95%CI | |
| No cancer | ||||||||
| Total patients | 0.61 | 0.59-0.63 | 0.03 | 0.03-0.04 | 0.56 | 0.55-0.58 | 0.15 | 0.12-0.17 |
| Anticoagulation | ||||||||
| Yes | 0.58 | 0.55-0.61 | 0.04 | 0.03-0.05 | 0.55 | 0.54-0.56 | 0.16 | 0.14-0.18 |
| No | 0.70 | 0.67-0.74 | 0.05 | 0.04-0.06 | 0.57 | 0.55-0.60 | 0.11 | 0.09-0.13 |
| Total cancer | ||||||||
| Total patients | 0.64 | 0.56-0.72 | 0.03 | 0.02-0.04 | 0.56 | 0.52-0.60 | 0.17 | 0.15-0.19 |
| Anticoagulation | ||||||||
| Yes | 0.64 | 0.54-0.74 | 0.03 | 0.02-0.05 | 0.56 | 0.52-0.61 | 0.18 | 0.16-0.20 |
| No | 0.51 | 0.36-0.65 | 0.05 | 0.02-0.07 | 0.48 | 0.39-0.56 | 0.15 | 0.12-0.19 |
| Active cancer | ||||||||
| Total patients | 0.61 | 0.50-0.73 | 0.05 | 0.03-0.08 | 0.55 | 0.48-0.62 | 0.19 | 0.16-0.22 |
| Anticoagulation | ||||||||
| Yes | 0.62 | 0.48-0.76 | 0.06 | 0.03-0.09 | 0.55 | 0.47-0.64 | 0.20 | 0.17-0.23 |
| No | 0.40 | 0.27-0.54 | 0.05 | 0.01-0.09 | 0.48 | 0.34-0.63 | 0.16 | 0.11-0.22 |
| Prior cancer | ||||||||
| Total patients | 0.66 | 0.56-0.77 | 0.02 | 0.01-0.03 | 0.56 | 0.51-0.60 | 0.17 | 0.14-0.19 |
| Anticoagulation | ||||||||
| Yes | 0.65 | 0.52-0.79 | 0.02 | 0.01-0.04 | 0.56 | 0.51-0.62 | 0.17 | 0.15-0.19 |
| No | 0.70 | 0.50-0.89 | 0.02 | 0.00-0.05 | 0.47 | 0.36-0.58 | 0.15 | 0.11-0.19 |
A total of 3846 patients died (38.9% from a cardiovascular condition) during a median follow-up of 4.9 years (interquartile range, 2.8-5.9 years). Embolic events were documented in 1464 patients (9.1%) and bleeding events in 5595 (34.8%; 1402 were major bleeding).
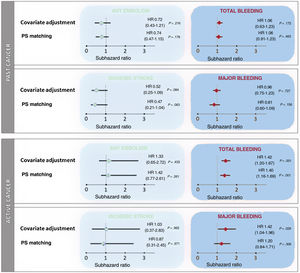
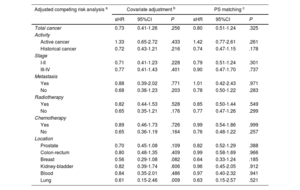
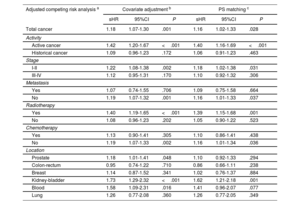
After adjustment for age, sex, CHA2DS2-VASc score, HAS-BLED score, and anticoagulation therapy, cancer was not associated with a higher risk of embolic events (sHR, 0.73; 95%CI, 0.41-1.26; P=.256). The absence of an association between cancer and embolic events was similar in anticoagulated (sHR, 0.73; 95%CI, 0.36-1.46; P=.372) and nonanticoagulated patients (sHR, 0.70; 95%CI, 0.28-1.79; P=.459). After analyzing by activity, stage, presence of metastases, use of chemotherapy or radiotherapy, and location, we found no significant association between cancer and embolic risk (table 3). However, in comparison with patients without cancer, patients with cancer had a higher bleeding risk (sHR for total bleeding, 1.18; 95%CI, 1.07-1.30; P=.001), which was consistent in both anticoagulated patients (sHR, 1.15; 95%CI, 1.03-1.29; P=.014) and nonanticoagulated patients (sHR, 1.28; 95%CI, 1.02-1.60; P=.031). The increased risk of bleeding was driven mainly by active cancer (sHR, 1.42; 95%CI, 1.20-1.67; P <.001). Radiotherapy was also associated with a higher bleeding risk (sHR, 1.19; 95%CI, 1.07-1.33; P=.002), with the most common cancer locations of patients treated with radiotherapy being the prostate (24.8%), the gastrointestinal tract (23.8%) and the urinary tract (11.1%). Information on cancer location and bleeding risk is shown in table 4. The lack of association between cancer and embolism, and the association between active cancer and bleeding, were maintained after the propensity score matching analyses (figure 2).
Association between cancer and embolic events based on 3 different adjusted analyses (reference group: patients without cancer)
| Adjusted competing risk analysis a | Covariate adjustment b | PS matching c | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Total cancer | 0.73 | 0.41-1.26 | .256 | 0.80 | 0.51-1.24 | .325 |
| Activity | ||||||
| Active cancer | 1.33 | 0.65-2.72 | .433 | 1.42 | 0.77-2.61 | .261 |
| Historical cancer | 0.72 | 0.43-1.21 | .216 | 0.74 | 0.47-1.15 | .178 |
| Stage | ||||||
| I-II | 0.71 | 0.41-1.23 | .228 | 0.79 | 0.51-1.24 | .301 |
| III-IV | 0.77 | 0.41-1.43 | .401 | 0.90 | 0.47-1.70 | .737 |
| Metastasis | ||||||
| Yes | 0.88 | 0.39-2.02 | .771 | 1.01 | 0.42-2.43 | .971 |
| No | 0.68 | 0.38-1.23 | .203 | 0.78 | 0.50-1.22 | .283 |
| Radiotherapy | ||||||
| Yes | 0.82 | 0.44-1.53 | .528 | 0.85 | 0.50-1.44 | .549 |
| No | 0.65 | 0.35-1.21 | .176 | 0.77 | 0.47-1.26 | .299 |
| Chemotherapy | ||||||
| Yes | 0.89 | 0.46-1.73 | .726 | 0.99 | 0.54-1.86 | .999 |
| No | 0.65 | 0.36-1.19 | .164 | 0.76 | 0.48-1.22 | .257 |
| Location | ||||||
| Prostate | 0.70 | 0.45-1.08 | .109 | 0.82 | 0.52-1.29 | .388 |
| Colon-rectum | 0.80 | 0.48-1.35 | .409 | 0.99 | 0.58-1.69 | .966 |
| Breast | 0.56 | 0.29-1.08 | .082 | 0.64 | 0.33-1.24 | .185 |
| Kidney-bladder | 0.82 | 0.39-1.74 | .606 | 0.96 | 0.45-2.05 | .912 |
| Blood | 0.84 | 0.35-2.01 | .486 | 0.97 | 0.40-2.32 | .941 |
| Lung | 0.61 | 0.15-2.46 | .009 | 0.63 | 0.15-2.57 | .521 |
95%CI, 95% confidence interval; PS, propensity score; sHR: subhazard ratio.
Association between cancer and bleeding events based on 3 different adjusted analyses (reference group: patients without cancer)
| Adjusted competing risk analysis a | Covariate adjustment b | PS matching c | ||||
|---|---|---|---|---|---|---|
| sHR | 95%CI | P | sHR | 95%CI | P | |
| Total cancer | 1.18 | 1.07-1.30 | .001 | 1.16 | 1.02-1.33 | .028 |
| Activity | ||||||
| Active cancer | 1.42 | 1.20-1.67 | <.001 | 1.40 | 1.16-1.69 | <.001 |
| Historical cancer | 1.09 | 0.96-1.23 | .172 | 1.06 | 0.91-1.23 | .463 |
| Stage | ||||||
| I-II | 1.22 | 1.08-1.38 | .002 | 1.18 | 1.02-1.38 | .031 |
| III-IV | 1.12 | 0.95-1.31 | .170 | 1.10 | 0.92-1.32 | .306 |
| Metastasis | ||||||
| Yes | 1.07 | 0.74-1.55 | .706 | 1.09 | 0.75-1.58 | .664 |
| No | 1.19 | 1.07-1.32 | .001 | 1.16 | 1.01-1.33 | .037 |
| Radiotherapy | ||||||
| Yes | 1.40 | 1.19-1.65 | <.001 | 1.39 | 1.15-1.68 | .001 |
| No | 1.08 | 0.96-1.23 | .202 | 1.05 | 0.90-1.22 | .523 |
| Chemotherapy | ||||||
| Yes | 1.13 | 0.90-1.41 | .305 | 1.10 | 0.86-1.41 | .438 |
| No | 1.19 | 1.07-1.33 | .002 | 1.16 | 1.01-1.34 | .036 |
| Location | ||||||
| Prostate | 1.18 | 1.01-1.41 | .048 | 1.10 | 0.92-1.33 | .294 |
| Colon-rectum | 0.95 | 0.74-1.22 | .710 | 0.86 | 0.66-1.11 | .238 |
| Breast | 1.14 | 0.87-1.52 | .341 | 1.02 | 0.76-1.37 | .884 |
| Kidney-bladder | 1.73 | 1.29-2.32 | <.001 | 1.62 | 1.21-2.18 | .001 |
| Blood | 1.58 | 1.09-2.31 | .016 | 1.41 | 0.96-2.07 | .077 |
| Lung | 1.26 | 0.77-2.08 | .360 | 1.26 | 0.77-2.05 | .349 |
95%CI, 95% confidence interval; PS, propensity score; sHR: subhazard ratio.
Association between prior cancer and active cancer with embolic and bleeding events after competing risk analysis according to 3 different adjustment methods, with death as a competing risk. Conventional covariate adjustment included age, sex, CHA2DS2-VASc score, HAS-BLED score, and anticoagulation therapy. PS matching adjustment analyzed the simple relationship between cancer and embolism after propensity score (PS) matching. Values of subhazard ratios (sHR) with their 95% confidence interval (95%CI) are reported, with the reference group being patients without cancer.
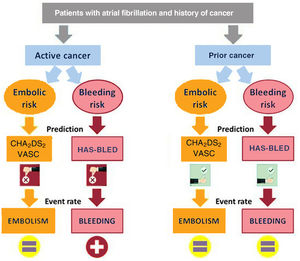
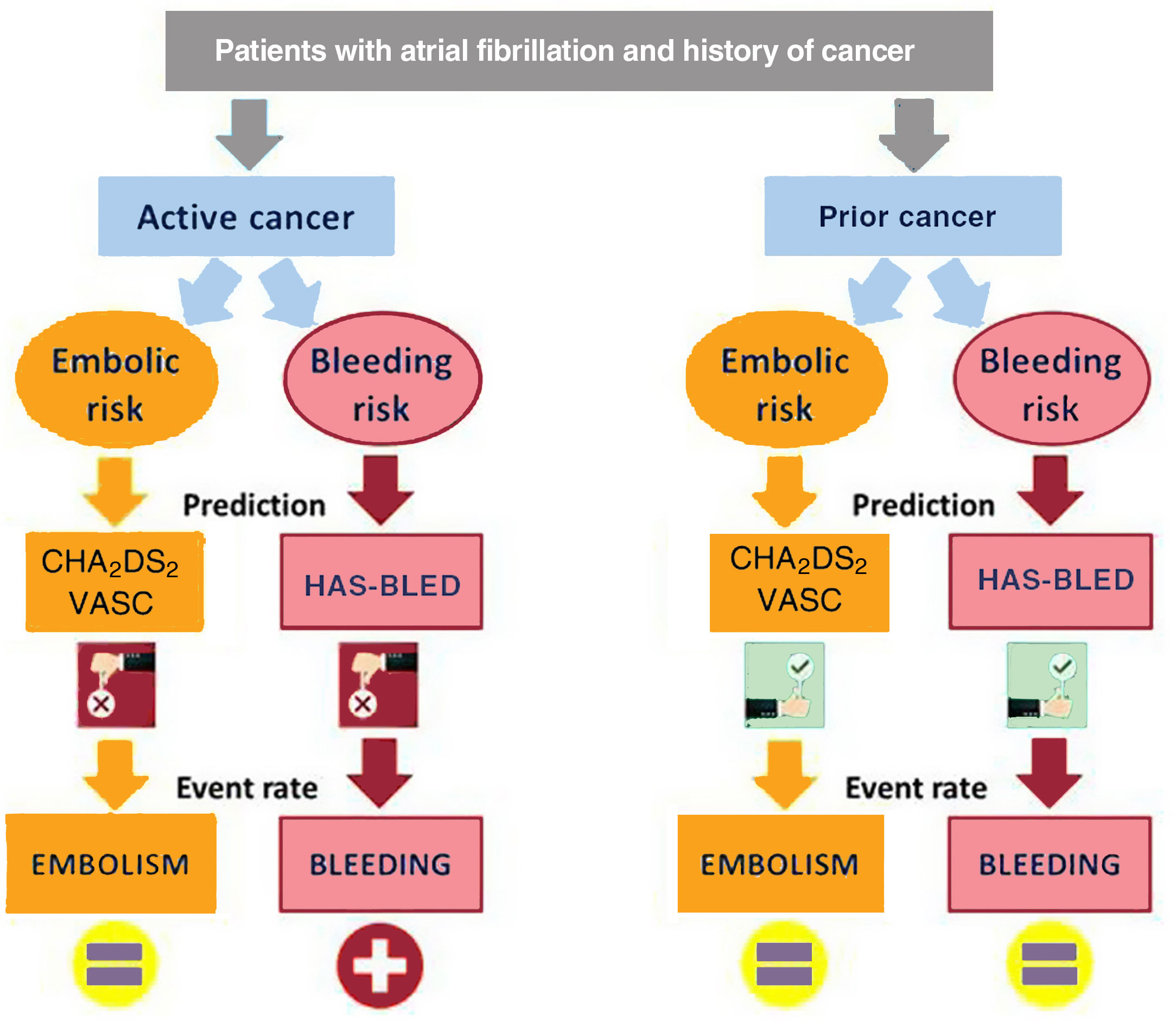
We analyzed the impact of the history of cancer on clinical outcomes in a large, heterogeneous, population-based sample of AF patients. The main findings of our study were as follows: a) The risk of embolism in AF patients with and without cancer was similar, although the risk of bleeding was higher in those with active cancer. b) Neither radiotherapy nor chemotherapy increased the risk of embolic events, although radiotherapy was associated with an increased risk of bleeding. c) The performance of both CHA2DS2-VASc and HAS-BLED scores to predict embolic and bleeding events, respectively, was poor in patients with cancer, especially in nonanticoagulated patients with active cancer (figure 3). d) The risks of both acute myocardial infarction and heart failure were similar between AF patients with and without cancer, although patients with cancer treated with chemotherapy had a considerably higher risk of heart failure than nononcological patients. e) No differences in cardiovascular mortality were found between AF patients with and without cancer. f) Anticoagulation was beneficial in both patients with and without cancer, with a similar embolic-hemorrhagic profile.
Cancer and risk of embolic eventsDespite huge advances in cardio-oncology in recent years, little is known about the risk of cardiovascular events in AF patients with cancer. Given coagulation cascade abnormalities and the chronic pro-inflammatory state that is secondary to several malignancies, it seems reasonable to expect a higher risk of both embolic and hemorrhagic events AF patients with an active cancer.5 In general populations without AF, cancer has been shown to be associated with a prothrombotic state, which increases the risk of arterial thromboembolic complications.12,13 However, while our results confirm the increased risk of bleeding in patients with cancer, we did not identify an increased risk of embolic events or acute myocardial infarction in our population of AF patients. While these findings may be surprising and contradictory to findings in patients without AF, they are consistent with the few studies carried out in AF patients with cancer. A prospective study of 294 989 Swedish patients hospitalized with a primary diagnosis of AF, including 71 882 with cancer, showed that neither a cancer diagnosis in general nor any specific cancer was associated with an increased risk of ischemic stroke.14 This finding is in line with the results of a Danish nationwide analysis of 68 119 hospitalized AF patients (11 855 with cancer), which showed that the risk of thromboembolic complications was nearly the same in patients with and without cancer.15 Our study extends the results beyond the hospital setting, including not only hospitalized patients, but also those from the outpatient setting. Moreover, our results are broken down according to cancer activity, extension, location, and therapy, with concordant results in all cases. However, despite multivariate adjustments and propensity score matching, we should be cautious because the percentage of anticoagulated patients was significantly lower in the group of cancer patients, which could influence our findings. In addition, unlike those of previous studies, our results are based on a competing risk analysis, regardless of anticoagulation therapy and CHA2DS2-VASc score.
Cancer and bleeding riskWe found an 18% increased risk of bleeding in AF patients with cancer. However, not all patients with cancer had an increased risk of bleeding. The relationship between cancer and increased bleeding risk was found only in patients with active cancer and in those treated with radiotherapy. This finding is consistent with those of prior studies, showing a higher incidence of bleeding in patients with active cancer due to the underlying malignancy, which caused invasive local growth and hemostatic abnormalities.14 In a nationwide cohort study of 2 435 541 AF patients, cancer increased the risk of major bleeding (HR, 1.27; 95%CI, 1.26-1.28).16 Bleeding in oncological patients can be related to quantitative and/or qualitative platelet abnormalities, which, in turn, may be caused by the underlying cancer itself or therapeutic interventions such as chemotherapy, radiotherapy, or surgery. In our study, radiotherapy—but not chemotherapy—proved to be a strong predictor of bleeding. These findings could have been influenced by the location of the cancers receiving radiotherapy, although the development of telangiectasias in irradiated mucosa has been reported to be the major pathological change leading to an increased risk of bleeding.17 Unlike other studies, we found no association between bleeding and cancer staging or the presence of metastases18; however, this could be because of the limited sample size.
Clinical implications of our resultsAnticoagulation therapy in patients with AF and cancer is particularly challenging because of the complex nature of this population.19 The uncertainty and lack of randomized treatment trials are reflected in the paucity of evidence-based recommendations for oncological patients with AF in clinical guidelines,20,21 although evidence is already available in observational studies on the benefit of oral anticoagulant treatment in patients with AF and active cancer.22 Our results have several clinical implications that deserve special attention. In our study, which included all patients from the health area with AF, the rate of anticoagulation in patients with cancer was high (74.8%). Other studies, based on patients from cancer centers, reported lower rates of oral anticoagulation in this population.23 It is likely that our findings were influenced by the different origin of the patients analyzed. A novel result of our study is that neither the CHA2DS2-VASc score nor the HAS-BLED score were good predictors of embolic and hemorrhagic events, respectively, in patients with active cancer. In a prior study, Pastori et al.24 found that the discrimination of the CHA2DS2-VASc discrimination was suboptimal in AF patients with cancer, with a c-statistic ranging between 0.56 and 0.61, depending on the cancer location. Although in our study the CHA2DS2-VASc score failed as a continuous variable for predicting embolic risk, as a categorical variable it might be useful. In this regard, our findings suggest than the presence of a single embolic risk factor (CHA2DS2-VASc=1 point) in patients with cancer already identifies patients with very high embolic risk who would need anticoagulation. However, further studies are needed to identify which patients with cancer and AF benefit from anticoagulation therapy. In relation to bleeding risk, patients with AF and cancer, particularly active cancer or cancer treated with radiotherapy, are at high risk of bleeding, regardless of the HAS-BLED score. Indeed, in our study, a patient with active cancer and HAS-BLED <3 had the same bleeding risk as a patient without cancer and HAS-BLED ≥ 3. In line with a previous study,25 we found that the discrimination of the HAS-BLED score for the prediction of combined clinically relevant major and nonmajor bleeding was poor, even in patients without cancer. New studies that independently analyze the risk of embolic and hemorrhagic events in cancer patients are needed to create new specific predictive risk scores for patients with cancer.
LimitationsThe observational nature of this study necessarily entails a series of caveats when interpreting its clinical implications. First, since there was no cancer screening protocol, we acknowledge that some patients may have had subclinical disease at the time of the AF evaluation and may have developed malignancy at a later date. Second, residual unmeasured confounding may have affected some findings. Third, the sample size and event rates were small, particularly in the active cancer group and in patients with metastatic cancer, thus limiting the robustness of the analysis and our ability to explore outcomes by cancer type or by chemotherapy agent. Fourth, only 99 patients with cancer had CHA2DS2-VASc ≤ 1 (16 with CHA2DS2-VASc 0 and 83 with CHA2DS2-VASc 1); therefore, it is difficult to reach firm conclusions in this group of patients. Finally, when assessing the impact of anticoagulation in patients with cancer, we must be cautious since our study is not a randomized clinical trial.
CONCLUSIONSIn AF patients, cancer might be associated with an increased risk of bleeding—especially in patients with active cancer and those treated with radiotherapy—but not with an increased risk of embolic events. Neither the CHA2DS2-VASc nor the HAS-BLED score showed good performance in predicting embolic and hemorrhagic events, respectively, in AF patients with active cancer.
FUNDINGThe electronic registry was funded in a unconditioned way by Daiichi Sankyo, Pfyzer-BMS, Bayer and Boehringer Ingelheim.
AUTHORS’ CONTRIBUTIONSAll the authors contributed to the creation of CardioCHUVI-AF registry, to the data analysis, and to the writing and revision of the present manuscript.
CONFLICTS OF INTERESTS. Raposeiras-Roubín has received honoraria for presentations and advisory boards from Amgen, Sanofi, AstraZeneca, Daichii, Pfyzer, Bayer, and Boehringer.
- -
In patients in sinus rhythm, cancer is associated with increased embolic and hemorrhagic risk. However, few studies have analyzed the influence of cancer on the real risk of embolic and hemorrhagic events, or the impact of risk scores for their prediction.
- -
Patients with AF and cancer have a higher bleeding risk than patients without cancer. However, their embolic risk is similar.
- -
Unlike patients without cancer, the CHA2DS2-VASc risk score fails to predict embolic events in patients with AF and cancer.
- -
The predictive ability of the HAS-BLED risk score was poor for AF patients with and without cancer.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.08.007