In the brain, amyloid-beta generation participates in the pathophysiology of cognitive disorders; in the bloodstream, the role of amyloid-beta is uncertain but may be linked to sterile inflammation and senescence. We explored the relationship between blood levels of amyloid-beta 1-40 peptide (Aβ40), cognition, and mortality (all-cause, cardiovascular, and heart failure [HF]-related) in ambulatory patients with HF.
MethodsBloodstream Aβ40 was measured in 939 consecutive patients with HF. Cognition was evaluated with the Pfeiffer questionnaire (adjusted for educational level) at baseline and during follow-up. Multivariate Cox regression analyses and measurements of performance (discrimination, calibration, and reclassification) were used, with competing risk for specific causes of death.
ResultsOver 5.1 ± 2.9 years, 471 patients died (all-cause): 250 from cardiovascular causes and 131 HF-related. The median Aβ40 concentration was 519.1 pg/mL [Q1-Q3: 361.8-749.9 pg/mL]. The Aβ40 concentration correlated with age, body mass index, renal dysfunction, and New York Heart Association functional class (all P < .001). There were no differences in Aβ40 in patients with and without cognitive impairment at baseline (P = .97) or during follow-up (P = .20). In multivariable analysis, including relevant clinical predictors and N-terminal pro-B-type natriuretic peptide, Aβ40 remained significantly associated with all-cause death (HR, 1.22; 95%CI, 1.10-1.35; P < .001) and cardiovascular death (HR, 1.18; 95%CI, 1.03-1.36; P = .02), but not with HF-related death (HR, 1.13; 95%CI, 0.93-1.37; P = .22). Circulating Aβ40 improved calibration and patient reclassification.
ConclusionsBlood levels of Aβ40 are not associated with cognitive decline in HF. Circulating Aβ40 was predictive of mortality and may indicate systemic aging.
Keywords
Heart failure (HF) is a growing epidemic with an important social and economic burden.1 Over the past 3 decades, treatment advances in HF have been made, mainly due to a better understanding of neurohormonal activation and its blockade. Presently, beta-blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers, and mineralocorticoid receptor antagonists are the cornerstone for HF treatment.2 Sacubitril/valsartan, the latest treatment in the HF armamentarium, has proved to have superiority over enalapril for treating HF patients with reduced ejection fraction.3 Sacubitril/valsartan belongs to a new class of dual action drugs that provide simultaneous inhibition of neprilysin (NEP) and blockade of the angiotensin receptor blocker.
Several lines of evidence suggest that diseases caused by age-related chronic “sterile” inflammation, such as heart disease and Alzheimer disease, may have common molecular pathways and share epidemiological, genetic, and environmental risk factors.4,5 The identification and monitoring of cellular senescence associated with such processes has not been conclusively achieved, but candidate circulating biomarkers may exist toward this goal. For example, generation of toxic amyloid-beta is one of the key events in the pathophysiology of Alzheimer disease.6–8 The amyloid-beta 1-40 peptide (Aβ40) is generated in the brain from amyloid precursor protein by β- and γ-secretase activities. Amyloid-beta 1-40 peptide is recognized as a proinflammatory peptide that acts through several mechanisms.9 In normal metabolism, Aβ40 is removed from the brain by multiple processes, including degradation by NEP.10
While circulating concentrations of Aβ40 have been linked with subclinical atherosclerosis and progression of arterial stiffness, independent of other conventional risk factors, along with prognosis,11 it is unclear whether bloodstream concentrations of this proinflammatory biomarker are associated with outcomes and/or cognitive decline in HF.
Accordingly, this study sought to evaluate the prognostic meaning of Aβ40 in a cohort of patients with chronic ambulatory HF untreated by NEP inhibition. In addition, we examined associations between bloodstream Aβ40 concentration, cognition assessed by the Pfeiffer questionnaire,12 and the following biomarkers: soluble NEP,13 soluble suppression of tumorigenicity-2 (ST2), and high-sensitivity C-reactive protein, a well-established inflammatory biomarker.
METHODSStudy PopulationAmbulatory patients treated at a multidisciplinary HF clinic between May 2006 and May 2013 were consecutively included in the study. The referral inclusion criteria and blood sample collection have been described elsewhere.13 In brief, patients were referred to the HF clinic by cardiology or internal medicine departments and, to a lesser extent, from the emergency or other hospital departments. The principal referral criterion was HF according to the European Society of Cardiology guidelines irrespective of etiology and at least 1 hospitalization for HF. Amyloid-beta 1-40 peptide and all other biomarkers were analyzed from the same blood sample, which was stored −80°C, without previous freeze-thaw cycles. All samples were obtained between 9:00 am and 12:00 pm. All participants provided written informed consent and the study was approved by the local ethics committee. All study procedures were conducted in accordance with the ethical standards outlined in the Helsinki Declaration of 1975, revised in 1983.
Cognitive AssessmentCognition was evaluated using the Pfeiffer questionnaire, a short mental status survey, in 802 patients at baseline (within 6 months of blood sampling) and in 405 patients during follow-up (median 3.4 years [2.1-6.1]). The test was considered diagnostic of cognitive impairment if the score was > 3. The patients’ educational levels were considered for scoring (± 1 scoring points relative to educational level), as previously reported.14
Follow-up and OutcomesAll patients were followed up at regular intervals, with additional visits, as required, in cases of decompensation.13 Patients who did not attend regular visits were contacted by telephone. The primary outcomes were all-cause death, cardiovascular (CV) death, and HF-related death. A death was considered of CV origin if it was caused by HF (decompensated HF or treatment-resistant HF, in the absence of another cause); sudden death (unexpected death, witnessed or not, of a previously stable patient with no evidence of worsening HF or any other cause of death); acute myocardial infarction (death directly related with acute myocardial infarction, whether due to mechanic, hemodynamic, or arrhythmic complications); stroke (associated with recent acute neurologic deficit); procedural (postdiagnostic or posttherapeutic death); or other CV causes (eg, rupture of an aneurysm, peripheral ischemia, or aortic dissection). Fatal events were identified from the clinical records of patients with HF, hospital wards, the emergency room, primary care physicians, or by contacting the patient's relatives. Furthermore, data were verified from the databases of the Catalan and Spanish Health Systems. Adjudication of events was performed individually by 2 of the authors (M. Domingo and J. Lupón).13
Biomarker AssaysAmyloid-beta 1-40Amyloid-beta 1-40 peptide was measured using the Aβ40 human ELISA Kit from DRG Instruments (Marburg, Germany). According to the manufacturer, the assay has no cross-reactivity with amyloid-beta 1-42 and amyloid-beta 12-28. The analytical sensitivity is 1.66 pg/mL, with no observed hook effect up to 96 000 pg/mL. The inter-assay coefficient of variation was 9.5% at 114 pg/mL and 7.9% at 585 pg/mL.
N-terminal Pro-B-type Natriuretic PeptideN-terminal pro-B-type natriuretic peptide levels were determined using an immuno-electrochemiluminescence method (Elecsys, Roche Diagnostics, Switzerland; n = 903). In the constituent studies in this report, the assay had an inter-run coefficient of variation ranging from 0.9% to 5.5%.
Soluble NeprilysinHuman soluble NEP was measured using a modified sandwich immunoassay (Aviscera Biosciences, Santa Clara, California, United States; Lot No. 20111893).13 For the positive control value of 1.4 ng/mL, the intra- and interassay coefficient of variation were 3.7% and 8.9%, respectively.
High-sensitivity C-reactive ProteinThe high-sensitivity C-reactive protein concentration was measured using a particle-enhanced turbidimetric immunoassay technique (Roche Diagnostics, Switzerland; n = 649). The functional sensitivity was 0.3mg/L, and the interassay coefficient of variation was < 8.4%.
Soluble Suppression of Tumorigenicity-2 AssaySoluble suppression of tumorigenicity-2 was measured using a high-sensitivity sandwich monoclonal immunoassay (Presage ST2 assay, Critical Diagnostics, San Diego, California, United States; N = 678). The soluble ST2 assay had a within-run coefficient of < 2.5%, a total coefficient of variation of 4%, and a limit of detection of 1.31 ng/mL.
Statistical AnalysisCategorical variables are expressed as percentages. Continuous variables are expressed as means ± standard deviation or medians [25-75 percentiles] according to normal or nonnormal distributions, respectively. Normality was assessed with normal Q-Q plots. Amyloid-beta 1-40 peptide values were log-transformed for analyses. Differences in Aβ40 concentrations among sexes, cognitive function, and HF etiology were assessed with the Mann-Whitney U test or Kruskal-Wallis test. Statistical differences (P value for trend) in Aβ40 concentrations across age strata (≤ 50, > 50 to ≤ 60, > 60 to ≤ 70, > 70 to ≤ 80, and > 80 years), New York Heart Association (NYHA) functional class (I, II, and III-IV), estimated glomerular filtration rate (eGFR) by Chronic Kidney Disease Epidemiology Collaboration formula (≥ 60, 30 to < 60, and < 30mL/min/1.73 m2), and body mass index (< 20.5, 20.5 to < 25.5, 25.5 to < 30, and ≥ 30kg/m2) were determined using the Spearman test. Correlations with Aβ40 were assessed with the Pearson test or Spearman's r ho (Pfeiffer questionnaire) as needed. Statistical differences between groups among categorical variables were assessed using the chi-square test. A multivariable lineal regression analysis was also performed to ascertain independent associations with Aβ40 levels.
To determine the independent predictive value of Aβ40 in HF, univariate and multivariable Cox regression analyses (backward step method) were executed for the defined endpoints. In all analyses involving CV and HF-related death, a competing risk strategy using the Gray method was adopted, considering non-CV as the competing event for CV death and other CV death and non-CV death for HF-related death. In multivariable analyses, a model with relevant clinical predictors (age, sex, ischemic etiology of HF, left ventricular ejection fraction, NYHA functional class, presence of diabetes mellitus, hemoglobin, serum sodium, eGFR, and treatment with beta-blockers and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker plus N-terminal pro-B-type natriuretic peptide) was developed and Aβ40 was included as an independent covariate. The proportionality and linearity assumptions were tested. To fulfill the assumption of linearity, the logarithmic functions of Aβ40 and N-terminal pro-B-type natriuretic peptide were used in the Cox models. Kaplan-Meier survival curves for all-cause death and cumulative incidence curves for CV and HF-related death were plotted. P values were obtained using log-rank and Gray method respectively
Comprehensive measurements of performance were used (discrimination and calibration) and the incremental value of adding Aβ40 to the model was explored by reclassification and discrimination improvement.
Statistical analyses were performed using the R (version 3.2.3) statistical package (Foundation for Statistical Computing, Vienna, Austria). A 2-sided P < .05 was considered significant.
RESULTSBlood levels of Aβ40 were measured in 939 patients with HF, who were consecutively enrolled from May 2006 to May 2013. The baseline characteristics of the cohort are shown in Table 1. During a mean follow-up period of 5.1 ± 2.9 years (6.9 ± 2.3 in alive patients), 471 patients died: 250 deaths from CV causes (53.1%), 171 from non-CV causes (36.3%), and 50 from unknown causes (10.6%). Among the known CV causes of death, refractory HF was the cause in 131 (52.4%) patients, sudden death in 55 (22%) patients, acute myocardial infarction in 23 (9.2%) patients, and other CV causes in 41 (16.4%) patients. Five patients were lost to follow-up and adequately censored.
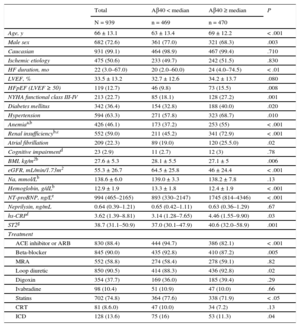
Clinical Characteristics and Treatment Relative to Amyloid-beta 1-40 Peptide Serum Levels Above or Below the Median
| Total | Aβ40 < median | Aβ40 ≥ median | P | |
|---|---|---|---|---|
| N = 939 | n = 469 | n = 470 | ||
| Age, y | 66 ± 13.1 | 63 ± 13.4 | 69 ± 12.2 | < .001 |
| Male sex | 682 (72.6) | 361 (77.0) | 321 (68.3) | .003 |
| Caucasian | 931 (99.1) | 464 (98.9) | 467 (99.4) | .710 |
| Ischemic etiology | 475 (50.6) | 233 (49.7) | 242 (51.5) | .830 |
| HF duration, mo | 22 (3.0–67.0) | 20 (2.0–60.0) | 24 (4.0–74.5) | < .01 |
| LVEF, % | 33.5 ± 13.2 | 32.7 ± 12.6 | 34.2 ± 13.7 | .080 |
| HFpEF (LVEF ≥ 50) | 119 (12.7) | 46 (9.8) | 73 (15.5) | .008 |
| NYHA functional class III-IV | 213 (22.7) | 85 (18.1) | 128 (27.2) | .001 |
| Diabetes mellitus | 342 (36.4) | 154 (32.8) | 188 (40.0) | .020 |
| Hypertension | 594 (63.3) | 271 (57.8) | 323 (68.7) | .010 |
| Anemiaa,b | 426 (46.1) | 173 (37.2) | 253 (55) | < .001 |
| Renal insufficiencyb,c | 552 (59.0) | 211 (45.2) | 341 (72.9) | < .001 |
| Atrial fibrillation | 209 (22.3) | 89 (19.0) | 120 (25.5.0) | .02 |
| Cognitive impairmentd | 23 (2.9) | 11 (2.7) | 12 (3) | .78 |
| BMI, kg/m2b | 27.6 ± 5.3 | 28.1 ± 5.5 | 27.1 ± 5 | .006 |
| eGFR, mL/min/1.73m2 | 55.3 ± 26.7 | 64.5 ± 25.8 | 46 ± 24.4 | < .001 |
| Na, mmol/Lb | 138.6 ± 6.0 | 139.0 ± 3.3 | 138.2 ± 7.8 | .13 |
| Hemoglobin, g/dLb | 12.9 ± 1.9 | 13.3 ± 1.8 | 12.4 ± 1.9 | < .001 |
| NT-proBNP, ng/Le | 994 (465–2165) | 893 (330–2147) | 1745 (814–4346) | < .001 |
| Neprilysin, ng/mL | 0.64 (0.39–1.21) | 0.65 (0.42–1.11) | 0.63 (0.36–1.29) | .67 |
| hs-CRPf | 3.62 (1.39–8.81) | 3.14 (1.28–7.65) | 4.46 (1.55–9.90) | .03 |
| ST2g | 38.7 (31.1–50.9) | 37.0 (30.1–47.9) | 40.6 (32.0–58.9) | .001 |
| Treatment | ||||
| ACE inhibitor or ARB | 830 (88.4) | 444 (94.7) | 386 (82.1) | < .001 |
| Beta-blocker | 845 (90.0) | 435 (92.8) | 410 (87.2) | .005 |
| MRA | 552 (58.8) | 274 (58.4) | 278 (59.1) | .82 |
| Loop diuretic | 850 (90.5) | 414 (88.3) | 436 (92.8) | .02 |
| Digoxin | 354 (37.7) | 169 (36.0) | 185 (39.4) | .29 |
| Ivabradine | 98 (10.4) | 51 (10.9) | 47 (10.0) | .66 |
| Statins | 702 (74.8) | 364 (77.6) | 338 (71.9) | < .05 |
| CRT | 81 (8.6.0) | 47 (10.0) | 34 (7.2) | .13 |
| ICD | 128 (13.6) | 75 (16) | 53 (11.3) | .04 |
Aβ40, amyloid-beta 1-40 peptide; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; BMI, body mass index; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration formula equation); HF, heart failure; HFpEF, heart failure with preserved ejection fraction; hs-CRP, high-sensitivity C-reactive protein; ICD, implantable cardioverter-defibrillator. LVEF, left ventricular ejection fraction; MRA, mineralcorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; ST2, soluble suppression of tumorigenicity-2.
Data are expressed as mean ± standard deviation, median (interquartile range) or No. (%).
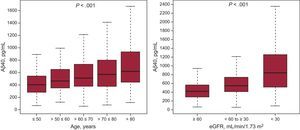
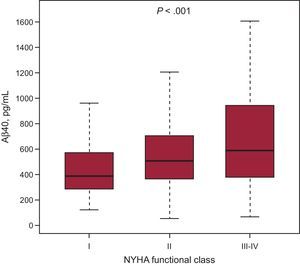
The median Aβ40 concentration was 519.1 pg/mL (361.8 to 749.9 pg/mL) and was weakly correlated with age (R = 0.19; P < .001), left ventricular ejection fraction (R = 0.08; P = .01), body mass index (R = 0.12, P < .001), and was slightly more correlated with renal function (eGFR; R = –0.39, P < .001). The Aβ40 values relative to age and eGFR strata are shown in Figure 1. Of note, Aβ40 levels were higher in women (median 562.8, [397.3-782]) than in men (496.4, [348.7-732.1]; P = .007). Amyloid-beta 1-40 peptide levels tended to be higher in patients with atrial fibrillation (median 564.0, [384.7-796.2]) than in those in sinus rhythm (500.6, [354.2-736.3], P = .056), and more patients with atrial fibrillation had Aβ40 levels above the median values (Table 1). A significant association was found between Aβ40 concentration and NYHA functional class (Figure 2); accordingly, Aβ40 moderately correlated with N-terminal pro-B-type natriuretic peptide (R = 0.30; P < .001). More weak correlation with high-sensitivity C-reactive protein (R = 0.11; P = .006) and ST2 (R = 0.15, P < .001) was found. Most importantly, given the role of NEP in the biology of Aβ40, we found no significant correlation between soluble NEP and circulating levels of Aβ40 (R = 0.04; P = .24).
In a multivariable lineal regression analysis, Aβ40 concentration was independently associated with eGFR (P < .001), left ventricular ejection fraction (P = .01) and N-terminal pro-B-type natriuretic peptide (P < .001), but not with sex (P = .21), NYHA functional class (P = .15), age (P = .07) or body mass index (P = .06).
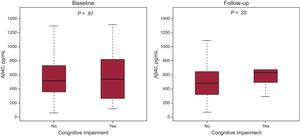
Amyloid-beta 1-40 Peptide and Cognition in Heart FailureBlood Aβ40 levels showed a weak correlation with Pfeiffer score both at baseline (Rho = 0.10, P = .004) and during follow-up (Rho = 0.10, P < .05). When the Pfeiffer questionnaire was used, cognitive impairment was present in 23 patients (2.9%) at baseline and in 9 patients (2.2%) during follow-up. No differences in blood Aβ40 levels were observed between patients with and without cognitive impairment (Pfeiffer questionnaire scoring adjusted for educational level) at baseline (P = .97) or during follow-up (P = .20) (Figure 3). If the patient's educational level was not considered for scoring, cognitive impairment was diagnosed in 29 patients (3.6%) at baseline and in 14 patients (3.6%) during follow-up. Again, no differences in blood Aβ40 levels were found between patients with and without cognitive impairment at baseline (P = .80) or during follow-up (P = .55).
Blood Levels of Amyloid-beta 1-40 Peptide and OutcomesAmyloid-beta 1-40 peptide levels were significantly higher in patients with all-cause death (median 586.2 pg/mL [405-851.9] vs 458.9 pg/mL [318.1-613.2], P < .001); CV death (median 605.2 pg/mL [418.3-892.7] vs 486.9 pg/mL [348-682], P < .001); and HF-related death (median 635.5 pg/mL [449.1-923.8] vs 496.7 pg/mL [353.7-718], P < .001). The diverging curves of survival and cumulative incidence of CV and HF-related death relative to Aβ40 median values are shown in Figure 4.
Survival curves relative to median blood Aβ40 concentration. A: Kaplan-Meier survival curves for all-cause death. B: cumulative incidence of cardiovascular death, taking into account other causes of noncardiovascular of death as competitive risk event. C: cumulative incidence of heart failure-related death, taking into account other cardiovascular and noncardiovascular causes of death as competitive risk event. Aβ40, amyloid-beta 1-40 peptide; HR, hazard ratio.
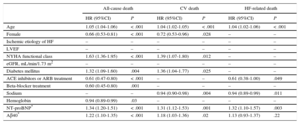
In the univariate analyses, Aβ40 levels, as a continuous variable, were significantly associated with all-cause death (hazard ratio [HR], 1.51; 95% confidence interval [95%CI], 1.37-1.66; P < .001), CV death (HR, 1.42; 95%CI, 1.25-1.62; P < .001) and HF-related death (HR, 1.41; 95%CI, 1.17-1.69; P < .001). In multivariable analyses, the addition of Aβ40 to a model that included relevant clinical predictors plus N-terminal pro-B-type natriuretic peptide was significantly associated with all-cause death (HR, 1.22; 95%CI, 1.10-1.35; P < .001), and CV death (HR, 1.18; 95%CI, 1.03-1.36; P = .02), but not with HF-related death (HR, 1.13; 95%CI, 0.93-1.37; P = .22) (Table 2). After addition of the inflammatory biomarkers high-sensitivity C-reactive protein and ST2 in the model, Aβ40 remained also significantly associated with all-cause death (P = .001) and CV death (P = .04), but not with HF-related death (Table 1 of the supplementary material).
Multivariable Cox Regression Analysis for Risk of All-cause, Cardiovascular and Heart Failure-related Death. For Cardiovascular Death and Heart Failure-related Death, the Competitive Risk Method was Used
| All-cause death | CV death | HF-related death | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | |
| Age | 1.05 (1.04-1.06) | < .001 | 1.04 (1.02-1.05) | < .001 | 1.04 (1.02-1.06) | < .001 |
| Female | 0.66 (0.53-0.81) | < .001 | 0.72 (0.53-0.96) | .028 | – | – |
| Ischemic etiology of HF | – | – | – | – | – | – |
| LVEF | – | – | – | – | – | – |
| NYHA functional class | 1.63 (1.36-1.95) | < .001 | 1.39 (1.07-1.80) | .012 | – | – |
| eGFR, mL/min/1.73 m2 | – | – | – | – | – | – |
| Diabetes mellitus | 1.32 (1.09-1.60) | .004 | 1.36 (1.04-1.77) | .025 | – | – |
| ACE inhibitors or ARB treatment | 0.61 (0.47-0.80) | < .001 | – | – | 0.61 (0.38-1.00) | .049 |
| Beta-blocker treatment | 0.60 (0.45-0.80) | .001 | – | – | – | – |
| Sodium | – | – | 0.94 (0.90-0.98) | .004 | 0.94 (0.89-0.99) | .011 |
| Hemoglobin | 0.94 (0.89-0.99) | .03 | – | – | – | – |
| NT-proBNP* | 1.34 (1.20-1.51) | < .001 | 1.31 (1.12-1.53) | .001 | 1.32 (1.10-1.57) | .003 |
| Aβ40* | 1.22 (1.10-1.35) | < .001 | 1.18 (1.03-1.36) | .02 | 1.13 (0.93-1.37) | .22 |
95%CI, 95% confidence interval; Aβ40, amyloid-beta 1-40 peptide; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; CV, cardiovascular; eGFR, estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration formula equation); HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association.
The model that included Aβ40 showed better global goodness-of-fit in all-cause (P < .001) and CV death (P = .02) and reclassification measured by continuous net reclassification index in all-cause (P = .001), CV (P < .001) and HF-related death (P = .01) (Table 2 of the supplementary material).
Table 3 of the supplementary material shows clinical characteristics and treatment relative to 2 periods of inclusion (2006-2009 vs 2010-2013). Although several differences were found between the 2 periods, there was no interaction between Aβ40 and the period of inclusion, and HR were very similar in separate multivariable analyses (HR, 1.29; [1.16–1.44] in the first period and HR, 1.32; [0.99–1.75] in the second).
Table 4 of the supplementary material and Table 5 of the supplementary material show multivariable survival analyses only in those patients with left ventricular ejection fraction < 50%. Amyloid-beta 1-40 peptide remained independently associated with all-cause death. The shows survival curves in that subgroup of patients relative to Aβ40 median values. Amyloid-beta 1-40 peptide blood levels above the median were significantly associated with higher all-cause mortality and higher cumulative incidence of CV and HF-related death than in the whole cohort.
DISCUSSIONWe investigated the clinical relevance of Aβ40 blood concentrations in a cohort of ambulatory patients with chronic HF, who were followed up for mortality over a median of 4 years. Amyloid-beta 1-40 peptide levels were detectable in all studied patients, and after adjustment for clinical predictors, Aβ40 was independently associated with all-cause death and CV death. The current findings suggest that high serum levels of Aβ40 may help identify HF patients at increased risk. Further, in our HF cohort (not treated by NEP inhibition) no association between bloodstream Aβ40 and cognitive decline was observed, which is of substantial importance, given concerns voiced regarding inhibition of NEP with emerging HF therapeutics.15
It is unclear why a higher Aβ40 concentration is an independent predictor of events in HF; the mechanisms involved are very likely multifactorial. The Aβ40 peptide may contribute to vascular aging. Heart failure represents the quintessential disorder of CV aging. Notably, arterial stiffness, the hallmark of arterial aging, was recently associated with Aβ40 in a cohort of patients with coronary heart disease.11 In HF, increased arterial stiffness leads to impaired ventricular-vascular coupling and has been associated with mortality.16 High Aβ40 levels may also play an important role in the pathogenesis of atrial fibrillation,17 which is present in almost a third of patients with HF. Further, our data indicate that the coexistence of HF and atrial fibrillation further increases Aβ40 levels. Finally, Aβ40 can function as an inflammatory stimulator to activate monocytes and trigger a marked increase in tumor necrosis factor and matrix metalloprotease-9 production, both involved in myocardial remodeling.18 A recent report found Aβ40 in the heart among patients with Alzheimer disease, indicating the important link between brain and heart disease.19
Bloodstream Aβ40 concentrations were associated with aging and eGFR, in accordance with previous reports,20 but Aβ40 correlated with events independently of these covariates. Further, Aβ40 levels increased as HF worsened, as assessed by New York Heart Association functional class. Decreased cerebral blood flow and neurohormonal activation may contribute to the dysfunction of the neurovascular unit, leading to Aβ40 accumulation in HF.21 Indeed, the blood levels of Aβ40 reported in the present study were over 3-fold higher than those found in patients with stable coronary artery disease.11
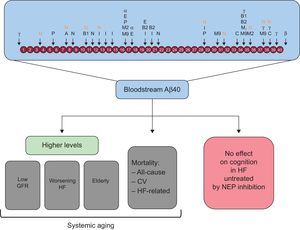
The clinical implications of the present data are noteworthy. The Aβ-degrading protease, NEP, has emerged as an important biotarget in HF. Here, soluble NEP was not associated with serum Aβ40 levels. On the one hand, circulating soluble NEP very likely only reflects a small fraction of all NEP present in the body in the form of a ubiquitous transmembrane receptor. On the other, amyloid-beta degradation is the responsibility of several proteases besides NEP, including angiotensin-converting enzyme (Figure 5).22 A recent report explored the effects of NEP inhibition on bloodstream Aβ40 when sacubitril/valsartan was administered over 14 days in healthy participants.23 From baseline to day 14, Aβ40 levels in plasma increased by 50% in the sacubitril/valsartan-treated group, reaching levels significantly higher than in the placebo group. In contrast, Aβ40 levels in cerebrospinal fluid remained unaltered in treated participants.23 To the best of our knowledge, there is no obvious or conclusive evidence suggesting that an isolated increase in bloodstream Aβ40 concentration results in or facilitates Aβ plaque formation in the brain or cognitive impairment. In fact, in the present study, blood levels of Aβ40 were not associated with cognitive impairment at baseline or during follow-up. Nevertheless, in view of the likely prolonged use of NEP inhibition for treatment of HF, clarification of the clinical relevance of NEP for amyloid-beta peptide clearance in HF is mandatory.2,24 It could be hypothesized that alternative proteolytic pathways may be activated with continued use of sacubitril/valsartan that compensate for NEP inhibition.25,26 It is acknowledged that the activities of several amyloid-beta degrading enzymes increase with age in Alzheimer disease, perhaps as a physiological response to minimize the buildup of amyloid-beta.10
Amyloid-beta can be processed by numerous proteases. Bloodstream Aβ40 may indicate systemic aging, but it is not associated with cognitive decline in HF. A, angiotensin-converting enzyme; α, α-secretase; Aβ40, amyloid-beta 1-40 peptide; B, β-site amyloid precursor protein-cleaving enzyme; β, β-secretase; B1, β-site amyloid-β-protein precursor cleaving enzyme 1; B2, β-site amyloid-β-protein precursor cleaving enzyme 2; C, cathepsin B; CV, cardiovascular; E, endothelin-converting enzyme-1 and -2; GFR, glomerular filtration rate; HF, heart failure; I, insulin-degrading enzyme; M2, matrix metalloprotease-2; M9, matrix metalloprotease; N, neprilysin; NEP, neprilysin; P, plasmin; γ, γ-secretase.
Although the study included a real-life HF population with different etiologies, the prominence of men with HF and reduced ejection fraction suggests that our results need to be further validated and should not be extrapolated to the general population with HF. In the near future, with the likely widespread use of sacubitril/valsartan in patients with HF and reduced ejection fraction, the prognostic value of Aβ40 may change. Alternative screening tests for cognitive impairment in older people are available. Nevertheless, the Pfeiffer questionnaire remains valuable27,28 and, in this study, it served as a proof of principle to explore the absence of an association between cognitive impairment and serum Aβ40. Further studies are required to assess whether the addition of a senescence biomarker such as Aβ40, together with conventional HF biomarkers, may provide further insight in HF prognosis, particularly in a real-life scenario with increasingly aging and co-morbid HF patients.
CONCLUSIONSThe present study demonstrated that bloodstream Aβ40 was independently associated with all-cause and CV mortality in a real-life cohort of patients with chronic HF not treated with NEP inhibitors. The present data suggest that bloodstream Aβ40 may be a valid indicator of systemic aging rather than cognitive impairment. Since Aβ40 is a substrate of NEP, the impact of NEP inhibitors on Aβ40 levels in HF remains to be determined.
CONFLICTS OF INTERESTNone declared.
- –
Diseases caused by age-related chronic “sterile” inflammation, such as heart disease and Alzheimer disease, may have common molecular pathways. Generation of toxic amyloid-beta is a key event in Alzheimer disease pathophysiology. Amyloid-beta 1-40 peptide is removed from the brain by multiple processes, including degradation by NEP. Recently, NEP has returned to center stage due to the positive effects observed with its inhibition by sacubitril/valsartan. There is uncertainty about the prognostic meaning of Aβ40 in patients with chronic ambulatory HF untreated by NEP inhibition, as well as the association between bloodstream Aβ40 concentration and cognition.
- –
Bloodstream Aβ40 levels were examined for the first time in a cohort of HF patients. Remarkably, Aβ40 was detectable in all studied patients, and after adjustment for clinical predictors, Aβ40 was independently associated with outcomes. Aβ40 may emerge as a valid senescence biomarker in HF.
- –
Further, this study ruled out a causative association between bloodstream Aβ40 and cognitive impairment in HF patients untreated with NEP inhibitors. Assessment of Aβ40 (in bloodstream and in the central nervous system) in patients treated with sacubitril/valsartan remains to be investigated.
We thank Beatriz González, Roser Cabanes, Margarita Rodríguez, Carmen Rivas, Nuria Benito and Alba Ros for data collection and invaluable work in the HF clinic. We also wish to acknowledge Redes Temáticas de Investigación Cooperativa en Salud, Red Cardiovascular (RD12/0042/0047; postdoctoral RIC fellowship), Red de Terapia Celular–TerCel [RD12/0019/0029], CIBER-cardiovascular (CB16/11/00403) and Ministerio de Economía y Competitividad (Juan de la Cierva, JCI-2012-14025).