Heart failure with preserved ejection fraction and reduced ejection fraction share a high mortality risk. However, differences in the rehospitalization burden over time between these 2 entities remains unclear.
MethodsWe prospectively included 2013 consecutive patients discharged for acute heart failure. Of these, 1082 (53.7%) had heart failure with preserved ejection fraction and 931 (46.2%) had heart failure with reduced ejection fraction. Cox and negative binomial regression methods were used to evaluate the risks of death and repeat hospitalizations, respectively.
ResultsAt a median follow-up of 2.36 years (interquartile range: 0.96-4.65), 1018 patients (50.6%) died, and 3804 readmissions were registered in 1406 patients (69.8%). Overall, there were no differences in mortality between heart failure with preserved ejection fraction and heart failure with reduced ejection fraction (16.7 vs 16.1 per 100 person-years, respectively; P=0794), or all-cause repeat hospitalization rates (62.1 vs 62.2 per 100 person-years, respectively; P=.944). After multivariable adjustment, and compared with patients with heart failure with reduced ejection fraction, patients with heart failure with preserved ejection fraction exhibited a similar risk of all-cause readmissions (incidence rate ratio=1.04; 95%CI, 0.93-1.17; P=.461). Regarding specific causes, heart failure with preserved ejection fraction showed similar risks of cardiovascular and heart failure-related rehospitalizations (incidence rate ratio=0.93; 95%CI, 0.82-1.06; P=.304; incidence rate ratio=0.96; 95% confidence interval, 0.83-1.13; P=.677, respectively), but had a higher risk of noncardiovascular readmissions (incidence rate ratio=1.24; 95%CI, 1.04-1.47; P=.012).
ConclusionsFollowing an admission for acute heart failure, patients with heart failure with preserved ejection fraction have a similar rehospitalization burden to those with heart failure with reduced ejection fraction. However, patients with heart failure with preserved ejection fraction are more likely to be readmitted for noncardiovascular causes.
Keywords
The risk of recurrent admissions in recently discharged patients with acute heart failure (AHF) remains prohibitively high.1–4 Readmissions due to worsening heart failure (HF) are associated with an increased mortality risk and account for a significant part of HF-related health care expenditure;2,5 moreover, noncardiovascular (non-CV) hospitalizations are frequent and also carry negative prognostic implications.3,6,7 Unfortunately, the identification of those HF patients at higher risk of recurrent admissions is an unmet clinical need.2,8
Traditionally, the “time-to-first” event approach has been the method used to evaluate the risk of adverse events in HF, including the risk of rehospitalization. From a methodological point of view, this type of analysis, although well-recognized, does not accurately reflect the hospitalization burden over the lifetime in HF, since this method ignores all the subsequent outcomes occurring after the first event.9 In recent years, some authors have argued in favor of replacing analyses of time-to-first readmission with longitudinal analyses that include all the events taking place during follow-up. This method would better quantify the burden of the disease and decrease type II errors.10,11
Heart failure with preserved ejection fraction (HFpEF) is present in up to nearly half of patients admitted to hospital with AHF.12 Patients with HFpEF exhibit a different range of comorbidities and overt pathophysiological differences to those with reduced ejection fraction (HFrEF).13–15 However, clinical presentation and mortality risk are similar in both entities.16–19 Whether HFpEF patients fare worse, similarly, or better than those with HFrEF in terms of rehospitalization risk over time remains unclear.
In this study, we sought to characterize the burden of repeat admissions over time following an admission for AHF, according to left ventricular ejection fraction (LVEF) status (HFpEF vs HFrEF).
MethodsStudy Group and ProtocolWe prospectively included a consecutive cohort of 2013 patients discharged for AHF in the cardiology department of a tertiary care teaching hospital (Hospital Clínico Universitario de Valencia, Spain) from January 1, 2004 to August 1, 2013. Acute heart failure was defined according to current Clinical Practice Guidelines.1 Patients with new-onset or acutely decompensated HF were included in the registry. By design, patients who died during the index hospitalization were not included in the final analysis. During the index hospitalization, data on demographics, medical history, vital signs, 12-lead electrocardiogram, laboratory and echocardiographic parameters, and drug use were routinely recorded using pre-established registry questionnaires. Left ventricular ejection fraction was assessed by 2-dimensional echocardiography in all patients during the index hospitalization (96±24hours after admission). Left ventricular ejection fraction was calculated by the biplane Simpson method. Two commercially available systems were used throughout the study, Agilent Sonos 5500 and ie33, (Philips, Massachusetts, United States). HFrEF and HFpEF were defined as LVEF<50% or ≥ 50%, respectively, based on previously established thresholds.1 Treatment with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, aldosterone antagonists, anticoagulants, diuretics, and other therapeutic strategies were individualized following established guidelines that were operating at the time the patient was included in the registry.
Follow-up and EndpointsThe incidence of all-cause, CV, non-CV, and HF-related rehospitalizations during follow-up were selected as the primary endpoints. Only unscheduled readmissions were included. The personnel in charge of endpoint adjudications were blinded to LVEF status. Each readmission during follow-up was labeled as follows: HF-related if it was due to worsening HF; CV-related if it was due to worsening HF, acute myocardial infarction, unstable angina, stroke or transient ischemic attack, cardiac arrhythmias, or peripheral artery disease. Otherwise, non-CV etiology was considered, and included cancer, infectious, gastrointestinal, renal, pulmonary, endocrine, urologic/gynecologic, and rheumatologic causes. Readmissions due to acute renal failure in the setting of worsening HF status were labeled as HF-related; otherwise they were considered as non-CV.
Ethical ConcernsThe study was prospectively designed, conformed to the principles outlined in the Declaration of Helsinki, and was approved by the institutional local review ethical committee. All patients gave informed consent.
Statistical AnalysisContinuous variables are expressed as mean±standard deviation (SD) or median (interquartile range [IQR]), whenever appropriate. Discrete variables were summarized as percentages. Baseline continuous variables were compared between LVEF<50% and LVEF ≥ 50% with the Student t test or rank-sum test as appropriate; discrete variables were compared with the chi-square test. The association between LVEF status with all-cause and CV mortality during follow-up was evaluated by Cox regression analysis. A descriptive analysis of rehospitalizations was performed by counting the number of hospitalizations during the entire follow-up. Crude incidence rates (expressed as the number of readmissions per 100 person-years) were calculated for each readmission endpoint (all-cause, CV, non-CV, and HF-related) across both LVEF categories. The independent association between LVEF and recurrent hospitalizations was assessed through a multivariable negative binomial regression (NBreg) analysis, and risk estimates were expressed as incidence rate ratio (IRR). There is concern about informative censoring in the field of HF. When this form of censorship is applied, an increase in rehospitalization risk can lead to an associated increase in the risk of subsequent death. It has been suggested that a way to empirically avoid this bias would be to consider any death occurring outside hospitalization as a new count (a readmission) in the NBreg model10,11 (the total numbers of all-cause, CV and HF deaths that occurred outside of any hospitalization were: 497, 320 and 234, respectively). All variables listed in Table 1 were evaluated for potential confounders. A backward stepwise selection, with Akaike information criterion as stopping criterion, was used to achieve parsimonious models. The covariates included in the multivariable clinical models for each primary endpoint were as follows: 1) All-cause rehospitalizations: age, sex, New York Heart Association class III or IV, previous HF admissions, hypertension, diabetes, former tobacco use, peripheral artery disease, dementia, chronic pulmonary artery disease, previous stroke, implantable cardioverter defibrillator carrier, previous myocardial infarction, atrial fibrillation interacting with heart rate, blood urea nitrogen, hemoglobin, sodium, N-terminal pro-B-type natriuretic peptide (NT-proBNP), and beta-blocker therapy; 2) CV rehospitalizations: age, sex, previous HF admissions, diabetes, former tobacco use, dementia, previous stroke, implantable cardioverter defibrillator carrier, previous myocardial infarction, atrial fibrillation interacting with heart rate, blood urea nitrogen, hemoglobin, sodium, NT-proBNP, and beta-blocker therapy; 3) Non-CV rehospitalizations: age, sex, New York Heart Association class III or IV, diabetes, former tobacco use, peripheral artery disease, personal history of malignant neoplasm, chronic pulmonary artery disease, previous stroke, implantable cardioverter defibrillator carrier, previous myocardial infarction, atrial fibrillation interacting with heart rate, blood urea nitrogen, hemoglobin, and beta-blocker therapy, and 4) HF-related rehospitalizations: age, sex, New York Heart Association class III or IV, previous HF admissions, diabetes, dementia, chronic pulmonary artery disease, implantable cardioverter defibrillator carrier, previous myocardial infarction, systolic blood pressure, atrial fibrillation interacting with heart rate, blood urea nitrogen, hemoglobin, sodium, NT-proBNP, beta-blocker therapy, and treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. The effect of LVEF status on the risk of rehospitalization assessed as a time-to-first event was also performed using Cox regression adapted for competing events. Cumulative incidence plots were adjusted for mortality as a competing event.20
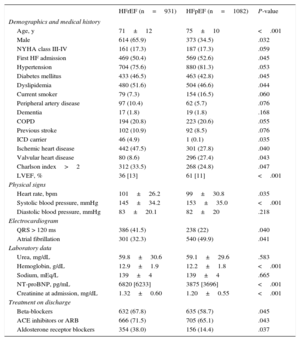
Distribution of Baseline Characteristics Between Heart Failure With Reduced Ejection Fraction Vs Heart Failure With Preserved Ejection Fraction
| HFrEF (n=931) | HFpEF (n=1082) | P-value | |
|---|---|---|---|
| Demographics and medical history | |||
| Age, y | 71±12 | 75±10 | <.001 |
| Male | 614 (65.9) | 373 (34.5) | .032 |
| NYHA class III-IV | 161 (17.3) | 187 (17.3) | .059 |
| First HF admission | 469 (50.4) | 569 (52.6) | .045 |
| Hypertension | 704 (75.6) | 880 (81.3) | .053 |
| Diabetes mellitus | 433 (46.5) | 463 (42.8) | .045 |
| Dyslipidemia | 480 (51.6) | 504 (46.6) | .044 |
| Current smoker | 79 (7.3) | 154 (16.5) | .060 |
| Peripheral artery disease | 97 (10.4) | 62 (5.7) | .076 |
| Dementia | 17 (1.8) | 19 (1.8) | .168 |
| COPD | 194 (20.8) | 223 (20.6) | .055 |
| Previous stroke | 102 (10.9) | 92 (8.5) | .076 |
| ICD carrier | 46 (4.9) | 1 (0.1) | .035 |
| Ischemic heart disease | 442 (47.5) | 301 (27.8) | .040 |
| Valvular heart disease | 80 (8.6) | 296 (27.4) | .043 |
| Charlson index>2 | 312 (33.5) | 268 (24.8) | .047 |
| LVEF, % | 36 [13] | 61 [11] | <.001 |
| Physical signs | |||
| Heart rate, bpm | 101±26.2 | 99±30.8 | .035 |
| Systolic blood pressure, mmHg | 145±34.2 | 153±35.0 | <.001 |
| Diastolic blood pressure, mmHg | 83±20.1 | 82±20 | .218 |
| Electrocardiogram | |||
| QRS > 120 ms | 386 (41.5) | 238 (22) | .040 |
| Atrial fibrillation | 301 (32.3) | 540 (49.9) | .041 |
| Laboratory data | |||
| Urea, mg/dL | 59.8±30.6 | 59.1±29.6 | .583 |
| Hemoglobin, g/dL | 12.9±1.9 | 12.2±1.8 | <.001 |
| Sodium, mEq/L | 139±4 | 139±4 | .665 |
| NT-proBNP, pg/mL | 6820 [6233] | 3875 [3696] | <.001 |
| Creatinine at admission, mg/dL | 1.32±0.60 | 1.20±0.55 | <.001 |
| Treatment on discharge | |||
| Beta-blockers | 632 (67.8) | 635 (58.7) | .045 |
| ACE inhibitors or ARB | 666 (71.5) | 705 (65.1) | .043 |
| Aldosterone receptor blockers | 354 (38.0) | 156 (14.4) | .037 |
ACE inhibitors, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Data are expressed as no. (%), mean±standard deviation or median [interquartile range].
A 2-sided P-value of<.05 was considered to be statistically significant for all analyses. All survival analyses were performed using STATA 14.1 (StataCorp. 2014. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP).
ResultsBaseline Patient CharacteristicsOf the 2013 patients included in this study, 1082 (53.7%) and 931 (46.2%) had HFpEF and HFrEF, respectively. The mean age was 72.8±11.2 years, 51% were women, 36.9% had ischemic etiology, and 48.4% had been previously admitted for AHF. The baseline characteristics of HFpEF and HFrEF patients are summarized in Table 1. Overall, important baseline differences were found between the 2 groups. Patients with HFpEF were older, more frequently female, and had a higher prevalence of hypertension and atrial fibrillation. Conversely, patients with HFpEF had a lower prevalence of ischemic heart disease, complete bundle branch block, and a lower mean Charlson index score. Of note, HFpEF patients had lower values of NT-proBNP and serum creatinine at admission (Table 1).
Risk of Mortality: Heart Failure With Preserved Ejection Fraction Vs Heart Failure With Reduced Ejection FractionAt a median follow-up of 2.36 years (IQR: 0.96-4.65), 1018 (50.6%) patients died. Of these, 480 (47.1%) were registered as CV deaths. Crude incidence rates for all-cause mortality were similar between the 2 groups: 16.2 events per 100 person-years in patients with HFpEF vs 16.7 per 100 person-years in those with HFrEF (log-rank test, P=.794). Patients with HFpEF showed lower rates of CV death. Crude incidence rates for CV mortality were 6.7 per 100 person-years in HFpEF and 9.0 per 100 person-years in HFrEF (log-rank test, P=.002). Kaplan-Meier all-cause and CV mortality curves for HFpEF and HFrEF are shown in Figure 1. Regarding specific causes of CV deaths, no differences were found between the 2 groups in myocardial infarction and stroke-related deaths (log-rank test, P=.134 and P=.452, respectively). Conversely, patients with HFpEF showed a trend to lower rates of sudden cardiac death (log-rank test, P=.098) and a significantly lower incidence of HF-related deaths (log-rank test, P=.015). Crude incidence rates for specific causes of CV death in the 2 groups are shown in Table 1 of the supplementary material.
A: Kaplan-Meier all-cause mortality curves for heart failure with preserved and reduced ejection fraction. B: Kaplan-Meier cardiovascular mortality curves for heart failure with preserved and reduced ejection fraction. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
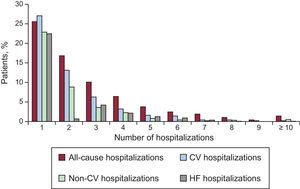
Throughout the follow-up, a cumulative total of 3804 readmissions were registered in 1406 (69.8%) patients. Of note, 552 (27.4%) and 219 (10.9%) patients had ≥ 3 or ≥ 5 readmissions during the course of the study, respectively. With regard to specific causes, 2233 CV-related readmissions were registered in 1085 (53.9%) patients, 1571 readmissions due to non-CV causes were registered in 806 (40.0%) patients, and 1589 HF-related readmissions were registered, resulting in 831 (41.3%) patients being readmitted due to worsening HF at least once. The distribution of the number of all-cause, CV, non-CV, and HF-related hospitalizations in the entire cohort is shown in Figure 2.
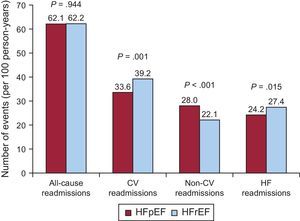
The crude incidence rates for rehospitalizations per 100 person-years according to LVEF are shown in Figure 3. Overall, there were no differences in the all-cause rehospitalization burden between HFpEF and HFrEF (62.1 vs 62.2 per 100 person-years, respectively; P=.944). Nevertheless, patients with HFpEF were less frequently readmitted for CV causes (33.6 vs 39.2 per 100 person-years; P=.001). Specifically, no significant differences were found for acute myocardial infarction (3.0 vs 2.9 per 100 person-years; P=.788) and stroke (2.2 vs 1.8 per 100 person-years; P=.316) readmissions. In contrast, significant differences were found for HF-related readmissions (24.2 vs 27.4; P=.015) and other CV causes (4.1 vs 7.1 per 100 person-years; P<.001). On the other hand, HFpEF patients showed a higher incidence rate for non-CV rehospitalizations (28.0 vs 22.1 per 100 person-years; P<.001).
Crude incidence rate of repeat all-cause, cardiovascular, noncardiovascular and heart failure-related hospitalizations in patients with preserved vs reduced ejection fraction. CV, cardiovascular; HF, heart failure. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
In an age- and sex-adjusted analysis, patients with HFpEF continued to show a higher risk of repeat non-CV hospitalizations, but also showed a slightly lower risk of repeat CV and HF-related admissions (Table 2). The effect on all-cause readmissions risk was neutral. After a fully multivariable adjustment, the risk of all-cause readmissions over time remained similar in the 2 groups. Thus, compared with HFrEF patients, those with HFpEF showed a similar risk of all-cause readmissions (IRR=1.04; 95%CI, 0.93-1.17; P=.461). Likewise, the adjusted risk of CV and HF-related rehospitalizations did not differ between patients with HFpEF and those with HFrEF (IRR=0.93; 95%CI, 0.82-1.06; P=.304, and IRR=0.96; 95%CI, 0.83-1.13; P=.677, respectively). In contrast, HFpEF patients continued to have a significant 24% increased risk of readmissions due to non-CV causes (IRR=1.24; 95%CI, 1.05-1.47; P=.012). Table 2 shows the unadjusted, age- and sex-adjusted, and fully-adjusted risk estimates for all-cause, CV, non-CV, and HF-related hospitalizations, as described above. Detailed multivariable models including all the covariates and their risk estimates are available in Tables 2–5 of the supplementary material.
Incidence Rate Ratios for Rehospitalizations in Heart Failure With Reduced Ejection Fraction Vs Heart Failure With Preserved Ejection Fraction
| IRR (95%CI) | P-value | Age- and sex-adjusted model IRR (95%CI) | P-value | Fully-adjusted model IRR* (95%CI) | P-value | |
|---|---|---|---|---|---|---|
| All-cause rehospitalizations | 1.06 (0.94-1.19) | .810 | 1.07 (0.96-1.21) | .215 | 1.04 (0.93-1.17) | .461 |
| CV rehospitalizations | 0.79 (0.69-0.91) | .001 | 0.74 (0.64-0.86) | <.001 | 0.93 (0.82-1.06) | .304 |
| Non-CV rehospitalizations | 1.28 (1.09-1.51) | .003 | 1.34 (1.13-1.59) | .001 | 1.24 (1.05-1.47) | .012 |
| HF hospitalizations | 0.85 (0.73-0.99) | .042 | 0.79 (0.67-0.92) | .003 | 0.97 (0.83-1.12) | .677 |
95%CI, 95% confidence interval; CV, cardiovascular; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IRR, incidence rate ratio.
Covariates for the fully-adjusted models are listed in the article and their risk estimates are available in Tables 2–5 of the supplementary material.
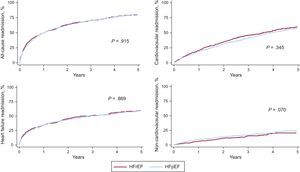
The risk of all-cause, CV- and HF-related readmission did not differ across LVEF status. Regarding non-CV readmission, patients with HFpEF displayed a trend to higher risk compared with patients with HFrEF. Cumulative incidence plots are shown in Figure 4.
Cumulative incidence plots of time-to-first all-cause, cardiovascular, heart failure-related, and noncardiovascular readmission in heart failure with preserved ejection fraction vs heart failure with reduced ejection fraction. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
In this study, we found that the cumulative hospitalization burden following an episode of AHF remained prohibitively high, irrespective of LVEF status. Although no overt differences were found in the risk of all-cause, CV, or HF-related readmissions, patients with HFpEF showed a moderate increase in the risk of non-CV rehospitalizations during long-term follow-up. To the best of our knowledge, this is the first study to examine the readmission burden of HFpEF and HFrEF through a state-of-the-art repeated events method. In addition, as reported in most the previous studies,16–19 mortality risk was similar in both patient groups.
The Rehospitalization Burden in Heart Failure: The Need for a More Detailed DescriptionHeart failure is a clinical syndrome characterized by frequent episodes of clinical decompensations, leading to a high hospitalization burden.2 Indeed, more than 50% of AHF patients return to hospital within 6 months of discharge.8 Once the patient is readmitted, the risk of further decompensations, disease progression, and subsequent mortality risk are substantially increased.2,21
Nevertheless, most of the evidence describing the risk of hospitalizations and its deleterious consequences in HF are from classic “time-to-first” event analyses, which ignore the vast information on subsequent events. This is a highly important topic, since recurrent events are a typical feature of HF disease progression. In line with other authors,9–11 we advocate for a better characterization of the morbidity burden in HF, including analysis of recurrent events over time instead of traditional “time-to-first event” methodology.22
To date, experience in repeated events analyses in HF is still limited, but there is increasing evidence of its usefulness. For instance, “time-to-first” event analysis in the SHIFT trial ignored 44% of the HF hospitalizations occurring during follow-up.23 In a cohort of 1077 new-onset HF patients, Dunlay et al.3 reported that 83% of them were hospitalized at least once, but interestingly 43% of the patients were hospitalized ≥ 4 times within 5 years of diagnosis. In our study, 70% of the patients were rehospitalized in the follow-up at least once, with up to nearly 30% of them having ≥ 3 readmissions. Moreover, a repeated events analysis can increase the ability to detect treatment effects to a greater extent than classic “time-to-first” event methodology, as has been the case in the CHARM, CORONA, SHIFT or EMPHASIS-HF trials.10,11,23,24 For instance, in the CORONA trial, rosuvastatin showed a modest protective effect in time-to-first readmission in patients with HFrEF, bordering on statistical significance; however, in a post-hoc analysis, rosuvastatin was associated with a greater statistically significant reduction in the risk of readmissions.11 Similarly, in our study, HFpEF was associated with a nonsignificant trend of non-CV rehospitalizations by time-to-first readmission analysis. Conversely, taking into account the entire burden of readmission events, HFpEF showed a 24% significantly higher risk of non-CV hospitalization. Nowadays, this methodology is implemented as a prespecified primary endpoint in ongoing clinical trials in HF, such as the PARAGON-HF study.25
Recurrent Hospitalizations: Heart Failure With Preserved Ejection Fraction Vs Heart Failure With Reduced Ejection FractionHeart failure is a clinically heterogeneous syndrome with a complex and multifactorial pathophysiology, but the most common distinction is based on LVEF status. HFrEF is a relatively well-known entity, with established treatment recommendations.1 Conversely, HFpEF is a complex disease closely linked to different extracardiac comorbidities, with a lack of specific treatments.15 Despite these overt differences between HFrEF and HFpEF, the mortality risk seems to be similar in both conditions.16–19 However, data on the risk and pattern of rehospitalizations in the 2 groups following a discharge for AHF are still scarce.
Prior studies have shown that the risk of readmission following an AHF discharge, in a classic “time-to-first” event analysis, is comparable between HFpEF and HFrEF, whether analyzing either short-term,16 or long-term events.26 However, the readmission pattern may differ. Previous studies suggest that patients with HFpEF are more likely to be readmitted for non-CV causes. Up to 44% of total readmissions in the patients included in the I-PRESERVE trial were due to non-CV causes.27 In the CHARM program, rates of non-CV rehospitalizations were higher in patients with LVEF ≥ 40% than for those with LVEF<40%.6 Interestingly, non-CV hospitalizations were associated with a subsequent mortality risk comparable to those related to CV causes.6 Similarly, in a large study by Cheng et al.,18 including 40 239 patients with chronic HF, the risk of non-CV readmissions was higher in HFpEF patients than in HFrEF patients. In contrast, rates of CV- or HF-related readmissions were higher in HFrEF patients.
Nonetheless, very few studies have examined the readmission burden between HFpEF and HFrEF accounting for repeated events analyses. Desai et al.6 found that LVEF was not an independent predictor of total rehospitalizations in new-onset HF patients. Chun et al.26 analyzed rehospitalizations among 8543 patients following a hospital discharge for new-onset AHF, and no overt differences in CV or HF-related readmission risks were registered between HFpEF and HFrEF. However, HFpEF patients had a modest increase in the adjusted risk for non-CV readmissions. That study used Cox regression analysis for recurrent events analyses9 and evaluated a cohort of patients included between 1999 and 2001, so it may not be possible to extrapolate the methodology and the above findings to the current time. In the present study, we go one step further, accounting for repeated events in the current era. We confirm that the overall morbidity burden, including all recurrent hospitalizations is similar across LVEF status. Likewise, and in line with prior reports, we found an increased risk of non-CV rehospitalizations in HFpEF. Accordingly, in previous studies, and compared with patients with HFrEF, those with HFpEF are more likely to die for non-CV reasons.28 Although an age- and sex-adjusted model showed that HFrEF predicted an excess of risk for CV and HF-related repeat admissions, a fully-adjusted model using traditional covariates failed to show this association.
We believe that the present results describe, in a more detailed fashion, the current excessively high morbidity burden of HF, irrespective of the presence or absence of systolic dysfunction. In addition, our data reinforce the idea of developing global and multidisciplinary management programs in HF beyond cardiac-specific treatments, with the aim of focusing not only on the prevention of CV or HF readmissions, but also on non-CV hospitalizations. This approach seems to be particularly important in HFpEF, a condition in which established therapies are still lacking, and comorbidities and non-CV readmissions play an important role.29,30
Study LimitationsThis study has some limitations. First, this is a single-center observational study in which there may be some particular circumstances and hidden biases influencing the pattern of hospitalizations. Second, we did not test for the influence of different causes or patterns of readmission on the risk of subsequent outcomes. Third, and under the assumption that the phenotype associated with HFrEF differed from that associated with HFpEF, we could argue that some residual confounding may have played a role in the lack of significance between HFrEF and etiology-specific readmissions. Fourth, the application of a repeated events methodology in the HF arena is relatively new and consequently some areas remain controversial.9 What is clear, however, is the statistical power advantage together with a better evaluation of the disease burden by using recurrent readmissions over the traditional “time-to-first event” endpoints.
ConclusionsRepeated events analysis showed that, following an admission for acute HF, the total rehospitalization burden over time was similar in patients with HFpEF and those with HFrEF. However, HFpEF patients were more likely to be readmitted for non-CV causes.
FundingThis work was supported in part by grants from Instituto de Salud Carlos III and FEDER, Red de Investigación Cardiovascular, Programa 7 (RD12/0042/0010).
Conflicts of InterestNone declared.
- -
The risk of adverse events is similar in patients with heart failure with preserved ejection fraction and those with reduced ejection fraction.
- -
The natural history of heart failure is characterized by recurrent hospital admissions, due to both cardiovascular and noncardiovascular causes.
- -
Repeated events analysis is, as currently stated by different authors, the most suitable method to deal with risk assessment of hospital readmissions in heart failure.
- -
Little is known about the burden of readmissions over time in patients with heart failure assessed by means of longitudinal repeated events analysis.
- -
Patients with heart failure with preserved ejection fraction exhibit a high burden of readmissions over time, similar to patients with heart failure and reduced ejection fraction.
- -
Patients with heart failure with preserved ejection fraction are more likely to be readmitted for noncardiovascular causes.