Improvements in early detection and treatment have markedly reduced cancer-related mortality. However survival not only depends on effectively cure cancer, but prevention, diagnosis and treatment of cancer-related complications is also needed. Cardiovascular toxicity is a widespread problem across many classes of therapeutic schemes, however scientific evidence in the management of cardiovascular complications of onco-hematological patients is scarce, as these patients have been systematically excluded from clinical trials and current recommendations are based on expert consensus. Multidisciplinary teams are mandatory to decrease morbidity and mortality from both cardiotoxicity and cancer itself. An excessive concern for the occurrence of cardiovascular toxicity, can avoid potentially curative therapies, while underestimating this risk, increases long-term mortality of cancer survivors. The objective of this consensus document, developed in collaboration of the Spanish Society of Cardiology, the Spanish Society of Medical Oncology, the Spanish Society of Radiation Oncology and the Spanish Society of Hematology, is to update the necessary concepts and expertise on cardio-onco-hematology that enable its application in daily clinical practice and to promote the development of local multidisciplinary teams, to improve the cardiovascular health of patients with cancer.
Keywords
The main cause of death of cancer survivors, together with second neoplasms, are cardiovascular diseases (CVDs).1 Although their treatment poses an enormous challenge, with onco-hematology treatments tripling the risk of cardiovascular events in the mid-to-long term,2–4 there is little scientific evidence on their management. To manage the treatment of cancer patients and minimize their cardiovascular toxicity, cardio-onco-hematology teams are composed of health care professionals involved in cancer patient care.5,6 The development of local prevention and early treatment protocols for cardiotoxicity can avoid early anticancer agent withdrawal, optimize health outcomes, and reduce costs. Thus, cardio-onco-hematology teams can ensure optimal clinical care and should help to coordinate continued research and medical education in the field.7
DEFINITION OF ONCO-HEMATOLOGY TREATMENT-RELATED CARDIOTOXICITYA variety of CVDs are caused by onco-hematology treatment-related cardiotoxicity and their diagnostic criteria are similar to those of the general population ().8 One of the most frequent but controversial complications is cancer therapeutics-related cardiac dysfunction (CTRCD). It is defined as a left ventricular ejection fraction (LVEF) reduction > 10% vs baseline with a LVEF lower than the normal limit. The European Society of Cardiology considers 50% to be the cutoff for normality,9 as in previous registries.10 However, in patients under treatment with anthracyclines11 or trastuzumab,12 a LVEF in the low-normal range (50%-55%) significantly increases the risk of CTRCD. Thus, and in accordance with the recommendations for cardiac chamber quantification,13 the American Society of Echocardiography and the European Association of Cardiovascular Imaging use 53% as the lower limit of normality.14 Both documents stress the importance of reproducible monitoring of LVEF, as well as the need for early initiation of CTRCD treatment to promote functional recovery.15,16
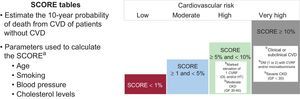
RISK EVALUATION AND CARDIOTOXICITY PREVENTION STRATEGIESEvaluation of Cardiotoxicity RiskCancer and the cardiovascular system share numerous risk factors.17 No prospective scoring systems are currently available for jointly evaluating cardiovascular risk (CVR) and cardiotoxicity risk, and traditional scales underestimate the risk associated with cancer treatment.3 Despite this limitation, CVR should be stratified using SCORE tables before anticancer therapy initiation (Figure 1),18 and physicians should evaluate the presence of factors determined in retrospective studies and registries to increase the risk of cardiovascular events during anticancer therapy (Table 1).9–12,14,19,20
Stratification of cardiovascular risk.18 CKD, chronic kidney disease; CVD, cardiovascular disease; CVR, cardiovascular risk; CVRF, cardiovascular risk factor; DL, dyslipidemia; DM, diabetes mellitus; GF, glomerular filtration; HT, hypertension. aA tool for SCORE calculation is available in the multimedia section of the Spanish Society of Cardiology website. bIndependently of the calculated SCORE (%), the presence of these factors is associated with a high or very high CVR.
Risk Factors for Ventricular Dysfunction in Patients Treated With Anticancer Drugs and Radiotherapy (if the Target Volume Includes at Least Some of the Heart)9–12,14,19,20
| CTRCD risk factors | Anthracyclines | Anti-HER2 | Anti-VEGF | Chest radiotherapy |
|---|---|---|---|---|
| Genetic factors | X | |||
| Cumulative dose | X | ≥ 35 Gy or ≥ 2 Gy/d | ||
| Female sex | X | X | ||
| < 15 or > 65 y | X | X | X | |
| Hypertension | X | X | X | |
| Ischemic heart disease | X | X | X | X |
| LVEF in the low range of normal (50%-55%) before treatment11,12 | X | X | ||
| History of heart failure/CTRCD | X | X | X | |
| Combined anticancer treatment* and chest radiotherapy | X | X | X | X |
| Renal failure | X | |||
| Obesity (BMI > 30) and sedentary lifestyle | X | |||
| Time since treatment | X |
anti-HER2, drugs blocking human epidermal growth factor receptor 2; anti-VEGF, vascular endothelial growth factor inhibitors; BMI, body mass index; CTRCD, cancer therapeutics-related cardiac dysfunction; LVEF, left ventricular ejection fraction.
The following strategy should be applied to all patients, independently of the planned treatment:
- •
Promotion of a heart-healthy lifestyle with a regular exercise program.21
- •
Identification and strict control of CVR factors (CVRFs) before, during, and after treatment. The therapeutic targets are summarized in Table 2 and are similar to those in the general population.18,22
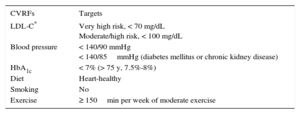
Table 2.Therapuetic Targets for Cardiovascular Risk Factors18,22
CVRFs Targets LDL-C* Very high risk, < 70 mg/dL
Moderate/high risk, < 100 mg/dLBlood pressure < 140/90 mmHg
< 140/85mmHg (diabetes mellitus or chronic kidney disease)HbA1c < 7% (> 75 y, 7.5%-8%) Diet Heart-healthy Smoking No Exercise ≥ 150min per week of moderate exercise CVRFs, cardiovascular risk factors; HbA1c, glycated hemoglobin; LDL-C, low-density lipoprotein-cholesterol.
The following strategy is recommended during the administration of potentially cardiotoxic treatments:
- •
Reduction of the direct cardiotoxic effect via the use of less cardiotoxic therapeutic regimens (liposomal formulations).9,23
- •
Use of cardioprotective agents in primary prevention.
- –
Dexrazoxane: reduces the risk of CTRCD due to anthracyclines but its use is controversial and atypical in Europe.9
- –
Beta-blockers (carvedilol and nebivolol): prevent a LVEF reduction and decrease the incidence of heart failure (HF) during trastuzumab and/or anthracycline therapy.24,25
- –
Angiotensin-converting enzyme (ACE) inhibitors: enalapril prevents LVEF deterioration in patients with troponin elevation during anthracycline therapy.26
- –
Combination therapies: the OVERCOME trial found reduced ventricular dysfunction (VD) and a lower incidence of death or HF in hematology patients under treatment with carvedilol and enalapril vs placebo.27 In patients with breast cancer, the PRADA study showed a cardioprotective effect of candesartan (but not candesartan and metoprolol combination) vs placebo.28
- –
Statins: high-potency statins have been shown in vitro and in retrospective studies to reduce cellular damage and HF risk in patients under treatment with anthracyclines.9,23,24
- –
Initial data23 support the use of aldosterone inhibitors in HF prevention.
- –
Systematic use of cardioprotective agents is not currently recommended for all cancer patients but they should be used in patients with previous heart disease and/or arterial hypertension (HT). They can be considered in primary prevention for patients with high or very high CVR or those who have previously received cardiotoxic drugs and require new anticancer treatment.9,18
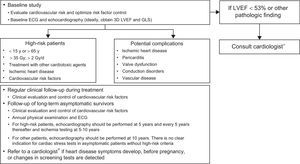
MONITORING AND DIAGNOSTIC ALGORITHMS FOR CARDIOTOXICITYClinical Follow-upIn addition to optimizing CVR and considering primary prevention strategies, cardio-onco-hematology teams should coordinate the monitoring of anticancer treatments for the identification and early treatment of possible cardiovascular complications (Table 3).7,9,14 The following patients should be referred to a cardiologist to evaluate the need for cardiac treatment and/or intensive monitoring9,14: those with poor control of CVRFs, significant alterations in imaging or biomarker studies, and/or heart disease symptoms.
Multidisciplinary Teams for Cardio-Onco-Hematology7,9,14
| Objectives of local Cardio-Onco-Hematology teams |
|---|
| 1. Streamline communication between professionals involved in the cancer treatment process |
| 2. Develop local protocols for prevention, diagnosis, and early treatment of cardiovascular complications |
| 3. Stratify cardiovascular risk of oncology patients |
| 4. Facilitate oncology treatment (eg, avoid repeated studies, minimize waiting lists) |
| 5. Avoid interruptions to anticancer treatment administration |
| 6. Record and analyze health and safety results |
| Cardio-Onco-Hematology clinic |
|---|
| 1. Optimize the control of risk factors and/or cardiovascular disease |
| 2. Optimize cardioprotective therapeutic measures |
| 3. Identify and treat at an early stage possible cardiovascular toxicities (maintain high clinical suspicion) |
| 4. Schedule follow-up for cancer survivors |
Biomarkers are tools for the early detection of myocardial damage.9,14,29,30 Troponin levels should be determined at baseline and before each cycle.31 An early (< 72 hours) troponin I (TnI) elevation (> 0.08 ng/dL) is detected in one-third of patients treated with anthracyclines.32 Persistent TnI elevation during cancer treatment identifies patients with worse cardiovascular prognosis who could benefit from ACE inhibitors to reduce CVD risk, avoiding the need for anticancer therapy withdrawal or modification.14,32 Less well-defined predictors of VD are the use of N-terminal pro-B type natriuretic peptide (NT-proBNP), a diagnostic marker of HF, and other biomarkers and/or other anticancer agents.31
Imaging TechniquesEchocardiography enables a general cardiac evaluation and is the technique of choice for serial measurement of LVEF. Two-dimensional (2D) LVEF shows poor sensitivity for the detection of minor changes in cardiac function because its variability is close to the diagnostic range of CTRCD (8%-11%).33 Contrast agent use and a systematic review of previous studies13 have improved the diagnostic accuracy. When available and used in experienced centers, 3-dimensional LVEF shows less variability (5.8%)34 and is the recommended echocardiographic technique for treatment monitoring (Table 4).9,14,35,36 Due to reduced availability, the use of cardiac magnetic resonance imaging, the reference standard for LVEF quantification, is limited to patients with unclear echocardiographic results.9,14,37 Isotopic ventriculography should not currently be considered for monitoring onco-hematologic treatments due to the risk associated wit ionizing radiation.9
Cardiac Imaging Techniques and Biomarkers in Anticancer Therapy Monitoring9,14,35,36
| Technique | Indication | Reference value | Advantages | Disadvantages |
|---|---|---|---|---|
| 2D-ECHO | Usual technique for monitoring LVEF | Symptomatic or asymptomatic LVEF reduction of > 10% vs baseline, with final LVEF < 53% | Availability Low cost Experience | Low reproducibility Image quality Established diagnostic technique for VD |
| 3D-ECHOa | Better reproducibility than 2D LVEF | Symptomatic or asymptomatic LVEF reduction of > 10% vs baseline, with final LVEF < 53% | Reproducibility | Availability Image quality Established diagnostic technique for VD |
| GLSa | Predicts HF | GLS reduction > 15% vs baselineb | Diagnostic technique for subclinical VD Reproducibility High negative predictive value | Availability Image quality Variability among vendors Absence of absolute normal value |
| CMRI | LVEF quantification if there are doubts with ECHO or discordance with other imaging techniques | LVEF < normal | Reproducibility Image quality Tissue characterization | Availability Technical limitations: obesity, claustrophobia, pacemaker, etc. |
| BMKs | Predict HF | TnI elevation > normal | Reproducibility Availability High negative predictive value | Variability among reagents No diagnostic or established range |
2D, 2-dimensional; 3D, 3-dimensional; BMKs, biomarkers; CMRI, cardiac magnetic resonance imaging; ECHO, echocardiography; GLS, global longitudinal strain; HF, heart failure; LVEF, left ventricular ejection fraction; TnI, troponin I; VD, ventricular dysfunction.
Once CTRCD has developed, initiation of HF treatment based on 2D LVEF alone does not guarantee functional recovery.38 New techniques, such as 2D speckle tracking, can identify myocardial damage at an even earlier stage by studying myocardial deformation (“strain”).39 At baseline, global longitudinal strain (GLS) improves the stratification of CTRCD risk vs 2D LVEF.40 During anticancer therapy, GLS reveals myocardial damage at an earlier stage and with less variability than LVEF (intraobserver, < 4%; interobserver, < 6%).41–45 Evaluation is recommended of relative changes in GLS, instead of absolute values, as well as the use of the same equipment and quantification software. Global longitudinal strain reductions > 15% from baseline indicate structural cardiac damage (stage B HF).9,14,46 Combination use of GLS and TnI improves their negative predictive value for VD and, thus, HF risk stratification.9,14
Monitoring Algorithms for Patients With Cancer Therapeutics-related Cardiac DysfunctionInitial AssessmentThe initial evaluation considers various therapeutic options and enables monitoring scheduling. It should include the following: a) medical history and physical examination (to rule out heart disease); b) electrocardiography (to rule out arrhythmias, signs of ischemia, or QTc interval abnormalities); c) structural and functional evaluation of the heart using echocardiography and biomarkers (); d) structured advice on heart-healthy lifestyle habits, and e) CVR stratification and treatment optimization for CVRFs and eventual heart disease; negative inotropic agents should be avoided.9,14
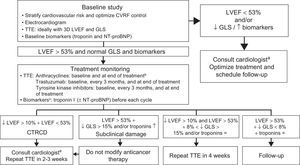
Treatment MonitoringThe objective is to use echocardiography to detect symptoms and ventricular function changes at an early stage. Biomarkers and/or GLS, depending on local experience and availability, improve risk stratification. Monitoring has traditionally differed according to whether patients receive type I or type II anticancer agents; however, patients rarely receive a single drug, which is why study frequency should be based on CVR, the therapeutic regimen, and local availability.9,14,29,30Figure 2 and Figure 3 are based on the LVEF < 53% criterion proposed by the American Society of Echocardiography and the European Association of Cardiovascular Imaging.14 This approach permits the early diagnosis of CTRCD, potentially avoiding any interruption to the anticancer therapy.
Monitoring algorithm for anticancer drug therapy.14 3D, 3-dimensional; CTRCD, cancer therapeutics-related cardiac dysfunction; CVRFs, cardiovascular risk factors; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B type natriuretic peptide; TTE, transthoracic echocardiography. aIdeally, a specialist cardio-onco-hematology clinic. bReevaluation of LVEF is recommended before treatment completion if the cumulative dose exceeds 240mg/m2. In these patients, the LVEF should be regularly monitored until the end of treatment. cIn patients with low cardiovascular risk and without history of cardiotoxic treatment, determination of troponin levels before each cycle reduces the number of echocardiograms required and limits their use to symptomatic patients or those with troponin elevation.
Monitoring algorithm for radiotherapy in patients whose target volume includes at least part of the heart.19 3D, 3-dimensional; ECG, electrocardiography; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction. *Ideally, patients will be referred to a specialist cardio-onco-hematology clinic.
Heart failure diagnosis is based on the presence of its typical symptoms and signs and echocardiographic alterations or an elevation in natriuretic peptides.16,47 If HF is present, physicians should adhere to the conventional treatment algorithms and rule out ischemic heart disease.16,47
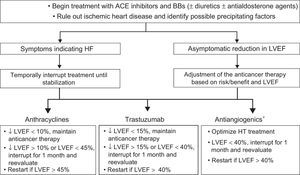
Beta-blocker and ACE inhibitors treatment of patients with reduced LVEF, whether symptomatic or asymptomatic, is required to avoid clinical HF and ventricular remodeling.11,16 Elevated levels of troponins and/or a GLS decrease > 15% with normal LVEF identify patients with asymptomatic structural damage (stage B HF) and at greater risk of progression to HF.14,46 Initial studies support the use of ACE inhibitors, with or without beta-blockers, to avoid withdrawal of potentially curative anticancer therapies.15,32,38,48
The indication for onco-hematology treatment withdrawal/delay should be individualized by weighing HF risk against that of cancer relapse/progression ( and Figure 4).9,14,29,49,50
Treatment and monitoring of patients receiving cardiotoxic agents and with a LVEF < 53%.9,14,16,29,49,50 ACE, angiotensin-converting enzyme; BBs, beta-blockers; HF, heart failure; HT, hypertension; LVEF, left ventricular ejection fraction. *See .
Heart failure treatment duration after LVEF normalization is controversial. Early withdrawal is not recommended. However, it can be considered, under strict surveillance, for asymptomatic patients without CVRFs and with normal parameters and stable ventricular function for more than 1 year (LVEF, GLS, TnI, and NT-proBNP).51 For patients with VD despite optimal treatment, the guidelines for HF should be followed regarding device implantation or heart transplant when permitted by the patient's vital prognosis.16,47
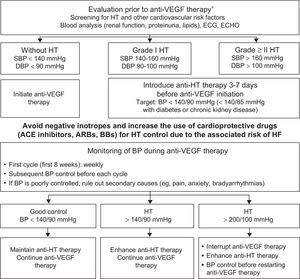
HypertensionHT is the most common comorbidity in cancer patients.8 Onco-hematology therapies cause HT via different mechanisms but the most frequently involved treatments are drugs that inhibit angiogenesis, interact with vascular endothelial growth factor (anti-VEGF agents), and decrease nitric oxide production.52 The incidence and severity of the resultant HT depend on the tumor, the drug, and the presence of other CVRFs ().53,54 Although HT caused by anti-VEGF therapy can predict a good tumor response, its control does not reduce therapeutic effectiveness55 but does avoid cardiovascular complications and treatment interruption.50 An initial assessment and close monitoring of blood pressure is recommended during the therapy by following the pharmacological and nutritional recommendations for the general population. The target blood pressure is < 140/90mmHg for patients with uncomplicated HT and < 140/85mmHg for diabetic patients and those with renal failure.56 Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers are the first-line drugs due to their ability to protect against HF development. If control is poor, amlodipine and aldosterone antagonists should be added. Thiazides should be used with caution due to the risk of hyperkalemia and QTc prolongation. Negative inotropes (diltiazem and verapamil) are discouraged due to the risk of HF and drug interactions (cytochrome P450 inhibitors increase sorafenib concentration) (Figure 5).57
Protocol for initiation, monitoring, and treatment of blood pressure in patients with indication for anti-VEGF therapy.9,46,57 ACE, angiotensin-converting enzyme; anti-VEGF, vascular endothelial growth factor inhibitor; ARBs, angiotensin II receptor blockers; BB, beta-blockers; BP, blood pressure; DBP, diastolic blood pressure; ECG, electrocardiography; ECHO, echocardiography; HF, heart failure; HT, hypertension; SBP, systolic blood pressure. *See .
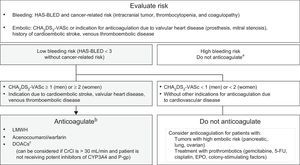
The most common sustained arrhythmia is atrial fibrillation. In patients without previous atrial fibrillation, it is typically triggered by cancer surgery, together with the use of certain drugs (). For patients receiving active anticancer therapy, the initial recommendation is rate control therapy.9,58 Anticoagulation use is guided by the CHA2DS2-VASc scale and bleeding risk (Figure 6).59,60
Indication algorithm for anticoagulation in patients with cancer-related atrial fibrillation.9,58–60 5-FU, 5-fluorouracil; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years (dual), diabetes mellitus, stroke (dual), vascular disease, age 65-74 years, and sex (female); CrCl, creatinine clearance; CYP, cytochrome P450; DOACs, direct oral anticoagulants; EPO, erythropoietin; HAS-BLED, hypertension, abnormal renal and liver function, stroke, history of or predisposition to bleeding, labile international normalized ratio, age > 65 years, and concomitant use of drugs or alcohol; LMWH, low-molecular-weight heparin; P-gp, P-glycoprotein. aFor patients with elevated bleeding risk and indication for anticoagulation not based on CHA2DS2-VASc score, the anticoagulation decision should be individualized. bAnticoagulant selection depends on clinical status, comorbidities, and possible interactions with the patient's anticancer therapy. cCurrently, there is no scientific evidence on its use in patients under active anticancer therapy.
In patients without structural heart disease, ventricular tachycardia risk depends on the effects of treatment on the transmembrane action potential (arsenic trioxide) or on coronary circulation (vasospasm due to 5-fluorouracil). Withdrawal should be considered, or its administration under hospital monitoring, of any drug if the QTc is > 500ms or increases more than 60ms from baseline.9
BradyarrhythmiasThe therapeutic approach is similar to that of patients without cancer, with consideration of pacemaker implantation for symptomatic bradycardias caused by taxanes and thalidomide, if the treatment has been clearly shown to improve survival.61
DevicesImplanted devices can be affected by radiotherapy. When the generator is located within the therapeutic field, its relocation can be considered to avoid interference. The clinical impact of radiotherapy on the device is directly proportional to the type and dose of energy administered and the degree of patient dependence on the pacing. Protective material, dose modeling, and assessment and follow-up protocols are vital (Figure 7).62 For patients with end-stage disease, antibradycardia pacing should not be modified unless it impairs quality of life but deactivation of antitachycardia therapies should be considered, if the patient agrees.63
Evaluation and follow-up protocol during RT administration to patients with implanted devices (pacemakers, defibrillators) whose target volume includes at least part of the heart. CTx, chemotherapy; HT, hormone therapy; ICD, implantable cardioverter defibrillator; PMs, pacemakers; RT, radiotherapy.
Although cancer can cause ischemic heart disease (IHD) via different mechanisms, the most frequent causes are sequelae of anticancer drugs and radiotherapy (Figure 8).9,19
There is no evidence that the diagnostic algorithms for the identification of IHD in patients with cancer should differ from those of the general population. The main recommendation is the detection and aggressive treatment of CVRFs and close monitoring of symptoms indicating coronary heart disease.9
Cancer Treatment in Patients With Ischemic Heart DiseasePatients with IHD under treatment with 5-fluorouracil, etoposide, bleomycin, vinblastine, bevacizumab, sorafenib, and taxanes should be monitored for the early detection of ischemic events.8,9 Drugs primarily linked to acute ischemia are 5-fluorouracil and its precursor, capecitabine (). There are no specific recommendations for prophylaxis, although 5-fluorouracil should not be administered for more than 3hours or combined with cisplatin.64 For patients who have had angina, nitroglycerin or calcium antagonist administration can prevent recurrence if there is no therapeutic alternative.64
Arterial thrombosis is a less common cause of ischemic events (< 1%).9 Its risk is related to disease extent, genetic predisposition, certain tumor types (pancreatic, ovarian, lung, and myeloma), and treatment with tyrosine kinase inhibitors (TKIs) and, to a lesser extent, cisplatin.54 In the absence of IHD, there are no absolute contraindications for the administration of any drug.9
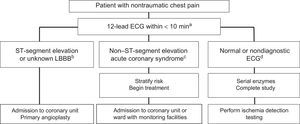
Management of Ischemic Complications of Cancer TherapyThere are no recommendations for prophylactic antiplatelet agents. For patients with angina, treatment optimization is recommended, as well as control of precipitating factors such as anemia. Patients with persistent symptoms are candidates for ischemia testing and eventual revascularization to enable therapy tolerance, depending on the risk/benefit ratio. Protocols for the evaluation of chest pain are similar to those for patients without cancer (Figure 9), with individualization of the revascularization strategy and antithrombotic regimen.9
Treatment algorithm for patients with chest pain in the emergency room. ECG, electrocardiography; LBBB, left bundle branch block. aA 12-lead ECG must be obtained and correctly interpreted within 10minutes, as well as serial troponin measurement. The ECG should be repeated if it is nondiagnostic or the pain disappears or reappears. bIn patients with persistent ST-segment elevation, primary angioplasty should be performed, as long as the patient's vital prognosis permits it and there are no hematological contraindications. cIn patients with non– ST-segment acute coronary syndrome, the risk must be stratified (GRACE scale) and the need for an invasive strategy must be assessed. If dual antiplatelet therapy is required, short courses are preferred and ticagrelor should be avoided in patients with active chemotherapy due to the risk of interactions with cytochrome P450 3A4. dIf the ECG is normal and serial enzymes are negative, ischemia testing should be performed according to the predefined and standardized protocol of the hospital.
Myocarditis is an uncommon complication of anticancer therapy ()65 that should be confirmed with cardiac magnetic resonance imaging. Its management follows the same criteria as in the general population.66 Immunosuppression can complicate prognosis, particularly in the presence of hemorrhagic myocarditis due to cyclophosphamide or tako-tsubo cardiomyopathy induced by 5-fluorouracil, cytarabine, or rituximab.67 Due to the risk of relapse, it is essential to reevaluate the cancer therapy and maintain cardioprotective efforts, at least until treatment completion.9
Acute pericarditis symptoms are uncommon and their management is similar to that of patients without cancer.68 They are related to chest radiotherapy, anthracyclines, cyclophosphamide, cytarabine, and bleomycin.9,19 Up to 20% of patients can acquire chronic effusive-constrictive forms years after the radiotherapy.19
Valvular Heart DiseasesRadiotherapy can damage the endocardium of valves, causing fibrosis, thickening, and dystrophic calcification.69 The degree of involvement is related to the time elapsed since radiotherapy.19 In autopsies, up to 81% of patients have evidence of valve damage, mainly mitral regurgitation, aortic stenosis, and calcification of the mitral-aortic intervalvular fibrosa.70 The probably of stenosis development increases 13.9 times for every 10 years since the radiotherapy.69 The treatment recommendations are similar to those for the general population, bearing in mind that radiotherapy increases the risk of cardiac surgery and that the type of lesion makes valve repair unlikely.71
Venous Thromboembolic DiseaseVenous thromboembolic disease (VTD), defined as deep vein thrombosis and/or pulmonary embolism, is 4 to 7 times more frequent in onco-hematology patients and has been documented in more than 20% of hospitalized patients with a malignancy.9 Factors associated with VTD are age, genetic predisposition, tumor extent and type (more common in lymphoma, myeloma, and pancreatic, ovarian, lung, stomach, and kidney cancer), and active treatment primarily with TKIs, immunomodulators (thalidomide, lenalidomide, and pomalidomide), 5-fluorouracil, cisplatin, and tamoxifen. These epidemiologic data justify prevention of VTD (Table 5).72,73
Prophylaxis and Treatment of Venous Thromboembolic Disease in Cancer Patients72,73
| Prophylaxis | |
|---|---|
| Oncology surgery | Before operation: |
| – LMWH 12hours before (high risk) or 2hours before (low risk) | |
| After operation: | |
| – Restart LMWH after 8hours, except in presence of active bleeding or high bleeding risk | |
| – Minimum duration of 7 to 10 days; 4 weeks recommended for patients with risk factors | |
| – If the bleeding risk is high or there are contraindications to LMWH: intermittent mechanical compression | |
| Cancer and reduced mobility | Thromboprophylaxis with LMWH is recommended |
| Antineoplastic chemotherapy | Systematic thromboprophylaxis is not performed |
| Thromboprophylaxis indicated (except with high bleeding risk in the HAS-BLED score) in: | |
| – Metastatic or locally advanced pancreatic cancer or lung cancer | |
| – Thalidomide or lenalidomide in combination with steroids/doxorubicin | |
| – Acute lymphoblastic leukemia under treatment with L-asparaginase (individualize) | |
| Treatment |
|---|
| Full-dose LMWH (if > 50 000 platelets/μL) (lower rates of thrombotic recurrence than with vitamin K antagonists) |
| Treatment duration: LMWH ≤ 3 months, 6 months desirable |
| Individualize treatment interruption: maintain LMWH until completion of curative treatment or resolution of risk factors for VTD recurrence |
| If venous thromboembolic disease recurs with LMWH, increase the dose and optimize anti-Xa levels. Evaluate vena cava filter in pulmonary embolism |
| Special situations in treatment of VTD |
|---|
| Brain tumors are not contraindications for LMWH |
| Begin postoperative thromboprophylaxis in neurosurgery |
| For severe renal failure (< 30 mL/min), adjust LMWH (anti-Xa levels) |
| Systematic prophylaxis is not recommended for central lines in the right jugular vein |
| Thrombosis associated with central lines: Full-dose LMWH, at ≤ 3 months (individualize catheter removal) |
| Current data on direct anticoagulants are scarce and there are no comparisons with LMWH |
LMWH, low-molecular-weight heparin; VTD, venous thromboembolic disease.
Pulmonary hypertension is an uncommon but serious complication that develops months or years after patient exposure to certain antineoplastic agents (TKIs, mainly dasatinib).9 The symptoms generally reverse upon treatment interruption. Cyclophosphamide can produce severe pulmonary hypertension due to pulmonary veno-occlusive disease.74 Diagnosis is based on clinical evaluation, echocardiography, and biomarkers (NT-proBNP). Pulmonary hypertension should be monitored every 3 to 6 months with echocardiography in patients under treatment with TKIs and should be suspected after significant changes in functional class. These patients require a multidisciplinary assessment to determine the best therapeutic strategy.9,75
Peripheral Vascular DiseasePeripheral vascular disease after radiotherapy mainly affects the arteries and capillaries and the lesions are distinct from those of classic atherosclerosis (less accumulation of lipids and macrophages, a marked endothelial thickening, destruction of the internal elastic lamina, necrosis of the vasa vasorum, and areas of bleeding within the plaque).9,19 Determination of its incidence is difficult due to the considerable heterogeneity of patient series but up to 70% of patients with head and neck tumors have carotid lesions 2 to 3 years after treatment.76
Administration of certain antineoplastic agents is associated with arterial thromboembolic phenomena () and early atherosclerosis in the distal part of the extremities (nilotinib or ponatinib).54 The prevention and treatment of atherosclerosis induced by anticancer agents or radiotherapy require close control of CVRFs, particularly in patients with prolonged survival.9,19
MONITORING ALGORITHMS FOR PROLONGED SURVIVALWhy Should We Perform Long-term Follow-up?About 11% of 40-year-old patients who survived a childhood neoplasm have a severe heart disease, generally HF, or require cardiovascular treatment.3 Systematic screening reduces the cumulative incidence of HF by 18%.77 Coordination of hospital teams with primary care is essential to meet the health care needs of long-term cancer survivors.7
Which Patients Require Follow-up?Most of the available information is derived from survivors of childhood cancer. Chow et al.78 developed and validated a HF risk score that classifies survivors into low risk (cumulative incidence of HF at age 40 years of 0.5%), medium risk (2.4%), and high risk (11.7%). The risk depends on the treatment received (cumulative dose of drugs/radiation and combined treatments) and the age at exposure. Follow-up is recommended for survivors who have received an anthracycline dose ≥ 250mg/m2 or ≥ 35Gy of chest radiotherapy or combined treatment with ≥ 100mg/m2 anthracyclines and ≥ 15Gy. Follow-up of low-risk survivors (anthracyclines < 100mg/m2 or < 15Gy) has not shown benefit.79
How and When to Follow-up?There is general agreement on the need for monitoring but not on how it should be performed. Clinical follow-up is vital and must rule out symptoms and signs of heart disease and provide structured advice on heart-healthy behavior and regular physical exercise (Table 2).18 Special attention should be paid to the increased risk of the development of CVRFs and metabolic syndrome.80,81
Echocardiography is recommended for monitoring ventricular function (generally LVEF; GLS can provide added information in certain patients4), although there are no prospective data on the frequency of monitoring. Armenian et al.79 proposed that echocardiography be performed in survivors of childhood cancer 2 years after treatment and every 5 years thereafter, as well as cardiovascular follow-up before and after pregnancy.
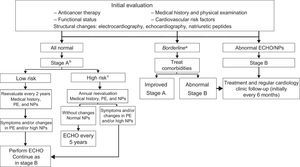
In breast cancer survivors, echocardiography is recommended at the end of anticancer therapy to permit follow-up planning (if the LVEF and GLS are normal, the risk of HF is lower).14 Pathological findings should prompt treatment and periodic monitoring by a cardiologist.9,16 A cost-effective proposal is the combined algorithm of Carver et al.,82 which evaluates symptoms, echocardiography, and natriuretic peptides (Figure 10).
Follow-up algorithm for patients with prolonged survival, modified from Carver et al.82 ECG, electrocardiography; ECHO, echocardiography; HF, heart failure; LVEF, left ventricular ejection fraction; NPs; natriuretic peptides; PE, physical examination. aMinor alterations in the ECG or intraventricular conduction disorder, nonsustained arrhythmias, or LVEF between 50% and 55%. bPatients with HF risk. cAny of the following conditions: age during treatment < 15 or > 65 years, female sex, any cardiac symptom or physical examination abnormality, cardiovascular risk factors, left ventricular dysfunction or previous heart disease, anthracyclines > 350mg/m2, chest radiation ≥ 35 Gy, combined treatment with anthracyclines and radiotherapy, premodern-era radiotherapy, follow-up > 10 years after treatment.
For asymptomatic adult survivors with high risk of heart disease due to radiation, echocardiography should be performed after 5 years, ischemia testing at 5 to 10 years, and a reassessment every 5 years (Figure 3).19
The main recommendations for the cardiovascular care of patients with cancer are summarized in Table 6.
Recommendations for the Cardiovascular Care of Cancer Patients
| 1 | Promote the development of local cardio-onco-hematology teams and the preparation of local prevention, diagnosis, and early treatment protocols for cardiotoxicity |
| 2 | Stratify cardiovascular risk and optimize the treatment of heart diseases and hypertension before initiation of anticancer therapy, avoiding negative inotropes |
| 3 | Recommend healthy lifestyle habits and optimal control of risk factors for all onco-hematology patients |
| 4 | Schedule anticancer therapy monitoring according to the plan and the risk of its causing cardiotoxicity |
| 5 | Evaluate the use of cardioprotective drugs for patients with high or very high cardiovascular risk or who during anticancer therapy monitoring show persistent biomarker elevation or significant reduction in global longitudinal strain |
| 6 | Initiate early treatment of ventricular dysfunction (regardless of symptom presence) and other cardiovascular complications according to the usual clinical practice guidelines |
| 7 | Avoid interruptions to anticancer treatment administration. Individualize the decision according to the risk/benefit of the treatment |
| 8 | Discuss with a cardio-onco-hematology team strategies to optimize cardiovascular and anticancer therapy of patients who develop cardiovascular toxicity |
| 9 | Schedule the follow-up of patients with prolonged survival |
| 10 | Establish criteria for health care quality, promote the creation of registries, and promote training programs and research studies |
Cardiovascular complications due to anticancer therapy are a growing clinical problem. The reversibility of myocardial damage in early phases has triggered interest in an approach based on prevention, monitoring, and early treatment strategies. This document unites for the first time experts from all Spanish scientific societies involved in the care of patients with cancer (Spanish Society of Cardiology, Spanish Society of Medical Oncology, Spanish Society of Radiation Oncology, and Spanish Society of Hematology) and summarizes the most important recommendations regarding the care of onco-hematology patients receiving potentially cardiotoxic anticancer treatments. Because the scientific evidence is scarce in many aspects, local multidisciplinary cardio-onco-hematology teams are essential to enable treatment optimization, investigation of the mechanisms underlying cardiovascular disease development, and promotion of cardiotoxicity research and registry creation.
CONFLICTS OF INTERESTNone declared.