Keywords
INTRODUCTION
Cardiovascular risk factors have long been studied in order to gain insight into the physiopathology, epidemiology and therapy of acute myocardial infarction (AMI). In this respect, recent advances in chronobiological methods have provided new knowledge regarding the behavior of rhythmical phenomena and their effects on cardiovascular risk.1-14
The majority of cardiovascular risk factors (which are well known) predispose the individual to developing acute coronary syndrome (in any of its different guises). Many of these factors can be used as prognostic indicators in patients who have suffered an AMI.15-17 The present paper pays particular attention to those that might influence the circadian variability of AMI.
A number of recent studies have suggested that cardiovascular events show temporal variability, and that episodes of myocardial ischemia are irregularly distributed over time, with an increased incidence during certain hours of the day, days of the week or even months of the year.4,18-28
Although myocardial ischemia can occur at any time of day, a circadian rhythm has been confirmed for the appearance of acute ischemic heart disease in the general population. Further, the MILIS18 study showed there to be a circadian pattern in the appearance of AMI. Other studies25,28-34 have confirmed these findings, although they show slight variations with respect to the beginning and end of peak incidence.
Some factors appear to modify the circadian rhythm of infarction in the general population, causing clinical AMI to be recognized over different time ranges.22,35,36 In patients with ischemic heart disease (understood here as any of its manifestations, e.g., angina, AMI, casual angiographic findings or findings appreciated from the results of functional tests, asymptomatic disease, etc), the rhythm of AMI symptom onset is similar to that of the general population, with a morning peak incidence. Patients who have suffered a previous AMI show a significantly increased number of episodes of ischemia during the evening (18:00 h-24:00 h); these also last significantly longer than usual.20
The ISIS-2 study reported the absence of a circadian rhythm in AMI symptom onset in diabetic patients,37 while others indicate circadian variations in the same type of patient with a peak incidence between 6:00 h and 12:00 h.9 In some studies, patients older than 70, smokers and women have shown a bimodal distribution of ischemic episodes with morning and evening peaks for AMI symptom onset.20,22
The present work attempts to determine the hours in which AMI symptoms begin in the general population and on the possible influence that different cardiovascular risk factors may have on the rhythm detected.
PATIENTS AND METHODS
The data examined in this work were those recorded by the ARIAM (Análisis del Retraso en el Infarto Agudo de Miocardio) project. Symptom onset times for AMI were recorded and an analysis performed to detect which variables might modify their periodicity.
The ARIAM project is a Spanish, multicenter study involving 119 centers around the country.38 Its database is an open registry and the information it contains is updated continuously. Among the data recorded is information on the main cardiovascular risk factors.39 Patients are "enrolled" consecutively from the moment that centers join the project. Currently, data are held on 86 000 patients; some 10 000 new patients are enrolled every year.
The study population consisted of 54 249 patients registered in the database between May 1994 and 2003. To study the influence of cardiovascular risk factors on the circadian rhythm of AMI onset, a subpopulation of 18 665 patients was selected whose files recorded the presence of these variables (information for these patients was obtained from the DATARIAMCAR database; this is associated with the general database containing the information for the above 54 249 patients).
All patients had a firm diagnosis of AMI. The diagnostic criteria for AMI initially used were those defined by the WHO and validated by the American College of Cardiology. After the Committee for the Redefinition of Acute Myocardial Infarction published its findings (consensus document of the Joint European Society of Cardiology/American College of Cardiology) in September 2000, the new criteria developed were gradually introduced at the participating centers; currently these are used for diagnosing every potential case of AMI.40,41
The influence of the following variables on the time of AMI symptom onset were recorded: sex, age (≥70 years and <70 years, in agreement with the majority of the literature), prior ischemic heart disease (in any of its clinical forms, diagnosed by anamnesis or electrocardiogram), condition at discharge (dead or alive), family background of ischemic heart disease (angina or AMI in men <55 years old and in women <60 years old), prior cerebrovascular accident (hemorrhagic or ischemic), arterial hypertension, diabetes mellitus, dyslipidemia, use of tobacco, and reinfarction.
Type of Study
This was a retrospective study in which the data recorded in the ARIAM database were analyzed. These data were processed in successive stages. Patient records were ordered chronologically. Data were recorded for the period 1 May 1994 until 31 October 2003.
Statistical Analysis
The study population was characterized using basic, descriptive statistical values (means for continuous variables and percentages or distribution of frequencies for discrete variables).
To analyze the influence of each variable on the circadian rhythm of AMI symptom onset, the study population was divided into different subgroups.
The goodness of fit of the different variables was examined by the χ² test: since each of the subgroups was defined by a single qualitative variable, attempts were made to determine whether the distribution of the AMI episode frequencies for each followed any rule, i.e., whether they fitted expected frequencies. The aim was to determine whether, for each variable, the frequencies were distributed according to a set pattern.
Circadian rhythms and their harmonics can be demonstrated using the cosinor method.42 This allows an adapted cosine curve to be constructed, in which several variables can be identified. The terms employed in chronobiological analysis when using the cosinor method are: a) rhythm: the periodicity of a pattern that oscillates in time: if the variation pattern is not periodic there is no rhythm; b) period: the time necessary to complete one cycle of a rhythmical phenomenon (the inverse of frequency); c) circadian: a rhythm with a period of approximately 24 h; d) frequency: the number of cycles completed in a given time; e) MESOR (midline estimating statistic of rhythm): the mean value between the maximum and minimum values of the sinusoidal curve; f) amplitude: the peak measurement of a rhythm above the mean threshold estimated by a mathematical function that measures the difference between the highest and lowest values on the cosine curve; g) acrophase: the time at which the maximum value established from the variable in the adapted cosine curve is measured; h) batiphase: the time at which the minimum value of the variable is established from the adapted cosine curve; and i) cosine analysis: the adaptation of a cosine curve to a rhythm by least squares regression.
Via the null amplitude test, the simple cosinor method can confirm the existence of a rhythm; via the amplitude-acrophase test it can be used to compare different rhythms.
In addition, the multiple and populational cosinor methods can be used to compare the rhythms of individuals from different subgroups.42 The limitations of this method are well known. Among the most serious is its low efficacy when used with data series that do not follow a reasonably sinusoidal distribution (such a distribution can be confirmed by the sinusoidality test). When this is the case, a fit is obtained in which the acrophase and batiphase are out of phase with the true maxima and minima respectively.9,43,44
To address the problems of the cosinor method, the system developed by Alberola et al45 was used. This was developed using the Matlab® platform and involves the use of the most important harmonics of the variables under study. In this way, the curves adjusted by the basic period and other harmonics better reproduce the effects of the different variables on the temporal distribution of AMI.43
Significance was set at α=5% for the existence of rhythmicity and for the comparison of rhythms.
RESULTS
Data for the study were all recorded between 1994 and 2003. The study population was composed of 54 249 patients registered between May 1994 and October 2003 in the ARIAM database. The analysis of modifiable cardiovascular risks was performed on a subpopulation of 18 665 patients.
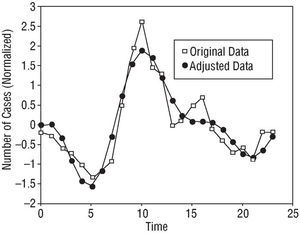
Table 1 shows the distribution of the total population, its general characteristics and the distribution of the different cardiovascular risk factors. Tables 2 and 3 show the results of the rhythmometric study. As a whole, the study population showed a circadian rhythm with respect to AMI symptom onset. Peak incidence, or acrophase, was detected at 10:07 h; the nadir or batiphase occurred at 4:46 h. The circadian pattern--the standard pattern--was sinusoidal (Figure 1).
Fig. 1. Circadian rhythm in the general population showing a standard pattern with a single incidence peak in the morning and a progressive decline over the rest of the day and night.
The subgroups resulting from the division of the population by sex and age also showed a circadian rhythm for AMI symptom onset, with the acrophase at around 10:00 h and the batiphase between 4:30 and 5:00 h. The patterns for these subgroups were similar to that seen in Figure 1 (Table 2).
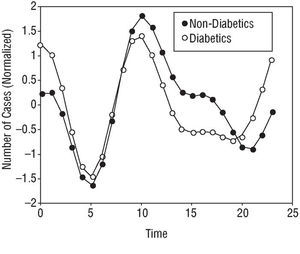
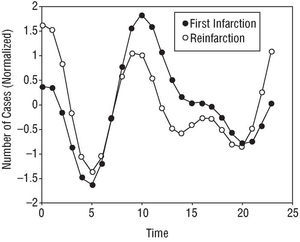
Some of the subgroups analyzed showed bimodal patterns in their AMI symptom onset curves. Patients with diabetes mellitus (Figure 2) and those who had suffered reinfarction (Figure 3) showed a circadian rhythm with a 12 h period; the reinfarction patients showed a nocturnal batiphase (0:22 h). This same pattern was observed in patients with prior ischemic heart disease.
Fig. 2. Circadian rhythm of the subgroup with diabetes, showing a bimodal pattern with two incidence peaks (morning and night-time) of different amplitude.
Fig. 3. Circadian rhythm of the subgroup suffering reinfarction, showing a bimodal pattern with two incidence peaks (morning and night-time) of different amplitude.
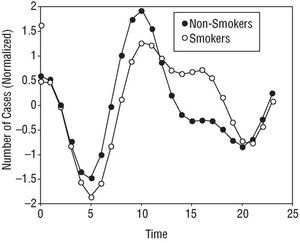
The smoker subgroup showed a lower evening than morning peak; this produced a plateau of AMI symptom onset incidence during the day. During the night, the incidence of AMI symptom onset in smokers and non-smokers was the same (Figure 4).
Fig. 4. Circadian rhythm of the smoker subgroup, showing an evening incidence plateau.
Having suffered a previous cerebrovascular accident, or having high blood pressure, dyslipidemia or family history of heart disease was found to have an influence on the circadian rhythm of AMI symptom onset, although the patterns produced were similar to the standard curve of Figure 1.
DISCUSSION
The periodicity of myocardial infarctions has been reported in numerous studies over the last twenty years.18-20,22,27,29,31,33,37,46-49
In the present patients (taken as a whole), a circadian rhythm was found for the onset of AMI symptoms. All the risk factor subgroups (previous cerebrovascular accident, family background of ischemic heart disease, diabetes, use of tobacco, dyslipidemia, hypertension, and reinfarction) showed a statistically significant clinical circadian rhythm for AMI symptom onset.
Some of the results obtained disagree with those of previous studies which report a lack of a circadian rhythm in certain populations. The methodology used to analyze rhythmicity in these previous studies, and the small size of their sample populations, may be the reason for this. For example, these earlier studies report no influence of high blood pressure on the circadian rhythm of AMI symptom onset. In the present study, high blood pressure did affect AMI symptom onset although the final curve was similar to the standard curve, with a morning peak.
Dyslipidemia affected AMI symptom onset in a manner very similar to high blood pressure. Again, the curve was similar to the standard curve for the entire population, with a morning peak.
The subgroup of patients who had suffered a previous cerebrovascular accident showed a circadian rhythm for AMI symptom onset. The curve was similar to the standard curve for the total population, although there was a more marked nocturnal incidence. As well as the possible influence of the drugs used in the treatment of these patients on the circadian rhythm of AMI symptom onset, an ischemic accident may, in some cases, affect the cerebral connections of the hypothalamic nucleus. The circadian rhythm of AMI symptom onset might therefore be modified, though this hypothesis has not been corroborated by electrophysiological or chronobiopathological studies.
The diabetic subgroup showed a circadian rhythm with a bimodal pattern involving one morning and one nocturnal peak (Figure 2). This might be explained by the autonomic dysregulation that diabetic patients suffer. The factors intimately associated with the pathogenesis of thrombotic episodes of AMI could be modified, both in terms of their circadian variability and intensity. The circadian rhythm of the heart rate and the nocturnal reduction in blood pressure could become attenuated, the morning threshold for platelet aggregation raised, fibrinolytic activity reduced, and daytime vascular thrombogenicity increased, etc.1,5,50
Behar et al29 and Zarich et al51 indicate that AMI symptoms in diabetic patients show a circadian rhythm with a single morning peak. In the work of Zarich et al,51 this circadian rhy thm is no longer seen in patients with serious dysregulation of the autonomous nervous system. Hjalmarson et al22 and Gilpin et al20 found a circadian rhythm with two peaks (morning and night-time) of similar intensity. The ISIS-2 (Second International Study of Infarct Survival) group also reported that diabetic patients had a circadian rhythm different to that of non-diabetics (although not significantly so).37 Finally, other authors have reported the absence of a circadian rhythm with respect to the onset time of AMI symptoms in patients with diabetes.37,52,53
The changes in the regulation of the autonomic nervous system experienced by diabetic patients may be one of the main reasons for the loss of a standard circadian rhythm for AMI symptom onset. The 2 incidence peaks might reflect this alteration, along with the greater sensitivity of the adrenergic receptors outside the morning maximum described in other subgroups. The frequent association between diabetes and other cardiovascular risk factors should also be remembered; frequent interactions may occur between diabetic phenomena and the pharmacological treatment of cardiovascular risks associated with the disease.
In patients with reinfarction, a significantly different circadian rhythm was found, with a bimodal incidence pattern in which the morning peak was attenuated and the evening peak as large as the morning peak (Figure 3). Since the patients with reinfarction met the inclusion requirement for prior ischemic heart disease, it is logical to think that their pharmacological treatments might be the cause of the changes seen in the presentation of the reinfarction. The results reported in this respect in the literature are contradictory. Hansen et al47 found a circadian rhythm for AMI symptom onset in a sample of 1872 reinfarction patients. The pattern found was similar to that reported in the present work, with both morning and night-time incidence peaks. Hjalmarson et al22 and Gilpin et al,20 however, found no such circadian rhythm in patients who had suffered a previous AMI (n=104); these patients showed a daily cycle that differed depending on whether a Q wave was present or not for the current AMI. Behar et al29 found a circadian rhythm in a sample of 477 patients, with a very notable morning peak of incidence. In all these papers, the methodology used was the comparison of incidence of AMI onset over 6 h intervals using the χ² test.
Figueras et al54 affirm that the onset of symptoms of a new infarction are subject to a circadian rhythm with an earlier incidence peak (between 0:00 h and 6:00 h) than that seen for first AMI, probably due to the anti-angina medication the patients received.
A comparison of the rhythms detected for first AMI and reinfarction shows them to have significant differences. The circadian rhythm of first AMI patients shows a standard pattern with a single morning peak followed by a progressive decline. Reinfarction patients, however, show a bimodal pattern, with circadian and a 12 h cycle, involving a larger nocturnal than morning peak (Figure 3). It may be that this difference in patterns is due to the pharmacological treatments used by patients who have already suffered an AMI.
The subgroup of smokers showed a circadian rhythm of AMI symptom onset different to that of non-smokers. The adjusted curve for the smokers shows a circadian rhythm of reduced amplitudes and a double peak incidence during the day, one in the morning and the other (less prominent) at around 16:00 h. The non-smokers showed a classic curve with a 24 h period and a single incidence peak followed by a progressive decline towards a nocturnal low point (Figure 4).
The results of Hjalmarson et al22 are in agreement with the present data with respect to the double peak of incidence shown by smokers. Kinjo et al,35 however, report a single nocturnal peak in young male smokers. In the present work, this subgroup of smokers showed the same double daily peak incidence and nocturnal low point as mentioned above (Figure 4). According to Tofler et al,13 tobacco might increase platelet aggregability. Certainly there is a relationship between smoking a cigarette and the activation of the sympathetic mechanisms of the autonomic nervous system. Spacing out smoking over the day might favor the attenuation of the morning peak and the appearance of an evening incidence plateau.
CONCLUSIONS
The onset time of AMI symptoms follows a sinusoidal curve with a morning peak and an early morning trough, conferring the appearance of these symptoms a circadian rhythm. This sinusoidal pattern and morning peak is maintained irrespective of sex or age in patients who have suffered prior ischemic heart disease or a prior cerebrovascular accident, and among those with family histories of ischemic heart disease (risk markers). Those with high blood pressure and dyslipidemia (modifiable factors) also show this pattern. However, patients with diabetes, who smoke, or who suffer reinfarction, show a double incidence peak in their curves.
Researchers of the ARIAM (Análisis del Retraso en el Infarto Agudo de Miocardio) Study Group
Advisory committee: M. Álvarez, J.J. Rodríguez, E. Torrado, A. García, (Málaga, Spain).
Collaborators: J. Canovas (Hospital General, Alicante); F. Guardiola, A. Roche (Hospital Virgen de los Lirios, Alcoi, Alicante); L. Gómez, A. Pérez (Clínica Benidorm, Alicante); J. Cardona, V. Madrid (Hospital General Marina Alta, Denia, Alicante); A. Mota (Hospital General, Elche, Alicante); J.A. Martín, J.A. Rodríguez (Hospital General, Elda, Alicante); F. Criado (Hospital del SVS, Benidorm, Alicante); J. Ortega, M. Martínez (Hospital Vega Baja, Orihuela, Alicante); G. Pérez (Hospital Universitario de San Juan, Alicante); F. Barredo, J.F. Martínez (Hospital Torrecárdenas, Almería); J. Córdoba (Hospital La Inmaculada, Huercal-Obera, Almería); L.J. Fierro, M. Ruiz (Hospital Comarcal de Poniente, El Egido, Almería); A. Montón (Hospital General Yagüe, Burgos); J. Armentia (Hospital Santiago Apóstol, Miranda de Ebro, Burgos); A. Sánchez, A. Gordillo (Hospital Puerta del Mar, Cádiz); P. Ramos, P. Cobos (Hospital Punta Europa, Algeciras, Cádiz); J. Arias, A. Rodríguez (Hospital de Jerez, Cádiz); J.L. García (Hospital Naval San Carlos, Cádiz); J.C. Rodríguez (Hospital General Puerto Real, Cádiz); A. Belenguer (Hospital General, Castellón); A. Canabal (Hospital General La Mancha-Centro, Alcázar de San Juan, Ciudad Real); F. Dios (Hospital Reina Sofía, Córdoba); A. Guerrero (Hospital Cruz Roja, Córdoba); C. de la Fuente, R. Toro (Hospital Infanta Margarita, Cabra, Córdoba); M. Ortiz (Hospital Valle de los Pedroches, Córdoba); P. Jiménez, J.M. Gulias (Hospital Juan Canalejo, A Coruña); J. González (Hospital Arquitecto Marcide, El Ferrol, A Coruña); L. Navarro, J.C. Pérez (Hospital General, Cuenca); E. Aguayo, A. Reina (Hospital Virgen de las Nieves, Granada); F. Barranco (Hospital Clínico, Granada); J.M. Mercade (Hospital Santa Ana, Motril, Granada); L. Rucabado, D. Montiel (Hospital General Ciudad de Jaén); A. Carrillo (Hospital Princesa de España, Jaén); A. de Molina (Hospital San Agustín, Linares, Jaén); A. Bartolomé (Hospital San Juan de la Cruz, Úbeda, Jaén); B. Álvarez, Z. Ferreras (Hospital del Bierzo, Ponferrada, León); F.J. Ochoa (Hospital San Millán, Logroño, La Rioja); E. Rodríguez, L. Martínez (Hospital Xeral-Calde, Lugo); J. Miquel (Hospital Universitario San Carlos, Madrid); E. Cereijo (Hospital de La Princesa, Madrid); J.D. García (Hospital del Aire, Madrid); J.L. Soria (Hospital Militar Gómez Ulla, Madrid); E. de la Fuente (Hospital Príncipe de Asturias, Alcalá de Henares, Madrid); F. del Nogal (Hospital Severo Ochoa, Leganés, Madrid); F.J. Goizueta (Hospital de Móstoles, Madrid); V. Gómez (Clínica Moncloa, Madrid); J. Ferriz, T. García (Hospital Regional Carlos Haya, Málaga); M.V. de la Torre, J. Merino (Hospital Clínico Universitario, Málaga); J.A. Ramos, A. Varela (Hospital Básico de Antequera, Málaga); J.I. Mateo (Hospital de la Serranía, Ronda, Málaga); A. García (Hospital La Axarquía, Vélez-Málaga, Málaga); J.A. Arboleda, R. Siendones (Hospital Costal del Sol, Marbella, Málaga); A. Sánchez, F.A. Jaime (Hospital La Arrixaca, Murcia); F. Felices, J. Segura (Hospital General Universitario, Murcia); J.A. Melgarejo, F.J. Gil (Hospital Virgen del Rosell, Cartagena, Murcia); S. Nicolás, J. Rodríguez (Hospital Comarcal Rafael Méndez, Lorca, Murcia); A. Carrillo, P. Jara (Hospital Morales Messeguer, Murcia); A. Manrique (Hospital Virgen del Camino, Pamplona, Navarra); F. Sos (Hospital García Orcoyen, Estella, Navarra); R. Rodríguez (Hospital Nuestra Sra. del Cristal-Sto. Cristo de Piñor, Ourense); V. López, C. Enríquez (Hospital Santa María Naí, Ourense); J.A. La Puerta, G. Sotres (Hospital de Cabueñes, Gijón, Asturias); J. López Messa, C. Berrocal (Hospital Río Carrión, Palencia); J.L. Martínez, A. País (Hospital General Montecelo, Pontevedra); D. Vila (Hospital Do Meixoeiro, Vigo, Pontevedra); J.J. Fambiño, J.E. Picón (Hospital Xeral, Vigo, Pontevedra); J.J. Cortina, P. Ancillo (Hospital General, Segovia); J. Maraví (Hospital Virgen del Rocío, Sevilla); J. Fajardo (Hospital Vigil de Quiñones, Sevilla); B. Maza (Hospital Ntra. Sra. de la Merced, Osuna, Sevilla); P. Medina (Hospital General, Soria); M. Rodríguez (Hospital Virgen de la Salud, Toledo); P. López (Hospital Ntra. Sra. del Prado, Talavera de la Reina, Toledo); J. Cuñat, R. Clemente (Hospital Universitario La Fe, Valencia); V. Segura (Hospital Doctor Peset, Valencia); M. García (Hospital Arnau de Vilanova, Valencia); C. Antón, R. Oltra (Hospital Clínico Universitario, Valencia); M. Roig (Hospital Militar, Quart, Valencia); J. López, J. Miñana (Hospital Francesc de Borja, Gandía, Valencia); V. Borillo (Hospital Lluis Alcanyis, Xátiva, Valencia); R. Calvo (Hospital General de Sagunto, Valencia); J.J. Sanz (Hospital Río Hortega, Valladolid); G. Hernando (Hospital de Galdakao, Vizcaya); A.C. Caballero (Hospital Virgen de la Concha, Zamora); E. Civeira (Hospital Clínico Lozano Blesa, Zaragoza); M.L. Centeno (Hospital de la Cruz Roja, Ceuta); F. Ríos (Hospital Comarcal, Melilla).
Correspondence: Dr. J.B. López Messa.
Unidad de Cuidados Intensivos. Hospital Río Carrión.
Avda. Donantes de sangre, s/n. 34005 Palencia. España.
E-mail: jlomessa@telefonica.net