Cell-free DNA (cfDNA) in ST-segment elevation myocardial infarction might originate from hyperactivated leukocytes at the coronary lesion. Our aim was to investigate the relationship between cfDNA and coronary reperfusion.
MethodsWe studied 116 patients treated with primary angioplasty using thrombus aspiration. Coronary (during aspiration) and peripheral (at the end of the procedure) blood samples were drawn for cfDNA, as well as high-sensitivity troponin T and myeloperoxidase quantification. The primary endpoint was no ST-segment resolution (STR) (≥ 70%) and the secondary endpoint was lack of final Thrombolysis In Myocardial Infarction flow 3 (TIMI 3).
ResultsST-segment resolution was achieved in 51 (44%) patients and TIMI 3 flow in 97 (84%). Patients without STR and TIMI 3 flow had a smaller peripheral-coronary cfDNA gradient (P = .02 and P = .04 respectively). A small cfDNA gradient (< 1.82 ng/mL) was associated with a higher rate of no STR (65% vs 30%; P = .001) and lack of TIMI 3 flow (21% vs 3%; P = .05). After multivariable adjustment, the small cfDNA gradient was predictive of no STR (OR, 4.50; 95%CI, 1.60-12.62; P = .004), while there was a nonsignificant trend for final TIMI 3 flow (P = .14). Cell-free DNA levels did not correlate with troponin T or myeloperoxidase.
ConclusionsA small peripheral-coronary cfDNA gradient, as an expression of high coronary cfDNA burden, is associated with no STR in acute myocardial infarction. Intracoronary cfDNA might reflect neutrophil activation. Whether this phenomenon contributes to thrombus aspiration failure requires further study.
Keywords
Several mechanisms have been proposed to explain microvascular damage and failed tissue reperfusion in the setting of ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous intervention.1 Hyperactivation of polymorphonuclear cells, leading to the release of their nuclear content into the extracellular space (cell-free DNA [cfDNA]), thus forming neutrophil extracellular traps (NETs), might play a role.2 Neutrophil extracellular traps constitute the scaffold for platelet aggregation and thrombosis, favoring microembolization and plugging in the microcirculation.3,4 Manual thrombus aspiration could not prevent this phenomenon, which might partially explain its lack of benefit evidenced in recent trials.5,6
Some studies have quantified cfDNA and NETs in STEMI.3,4,7–10 However, these studies consisted of small patient cohorts and their results were not adjusted for clinical and procedural variables. The present study measured intracoronary and peripheral cfDNA during primary percutaneous intervention using thrombus aspiration in a series STEMI. We aimed to analyze the association between cfDNA and coronary reperfusion after adjusting for well-known clinical and procedural predictive factors. In addition, the relationship between cfDNA and myeloperoxidase and high-sensitivity cardiac troponin T levels was also analyzed.
METHODSStudy DesignThis study had a prospective design and included a total of 116 patients with STEMI, recruited from April 2014 to September 2015 from 2 Spanish institutions (the University Clinic Hospital in Valencia, Spain and the Clinic Hospital in Barcelona, Spain). All the participants were treated with primary percutaneous intervention and were deemed suitable for manual thrombus aspiration because of their large angiographic thrombus burden. Inclusion criteria were STEMI within the first 12hours of pain onset and Thrombolysis In Myocardial Infarction (TIMI) thrombus grade 4 (a definite thrombus with the largest dimension ≥ 2 vessel diameters) or 5 (total occlusion) in the diagnostic coronary angiogram. Exclusion criteria were acute myocardial infarction (MI) secondary to stent thrombosis and culprit lesion characteristics considered inappropriate for use of the aspiration catheter (marked tortuosity or ostial or very distal lesions). All patients signed the corresponding written informed consent form for their participation in the study and the study was approved by the Ethics Committees at both centers.
Patient ManagementAn initial electrocardiogram was performed before the procedure in the catheterization room, and this was considered the baseline trace. The radial approach with 6-Fr sheaths and 6-Fr guiding catheters was used in nearly all the participants. All the patients were treated with heparin and aspirin; adjunctive antithrombotic treatment (P2Y12 receptor inhibitors and abciximab) and the primary percutaneous intervention technique were decided on an individual basis by the interventional cardiologist. At least 4 thrombus aspirations were recommended with the aim of restoring coronary flow and directly implanting the stent, if feasible. Intracoronary blood samples from the aspiration, as well as blood samples from the radial sheath after removal of the guiding catheter at the end of the procedure, were collected. Peripheral and coronary samples were not taken simultaneously because the radial sheath lumen was blocked by the guiding catheter during the primary percutaneous intervention. The time delay between blood samples withdrawn was recorded. One hour after termination of the procedure, the electrocardiogram was repeated and compared with the baseline trace. Data from a total of 37 clinical, electrocardiogram, angiographic, and procedural variables were collected for each patient (Table 1). The electrocardiogram analyses were all carried out by the same investigator who was blinded to the primary percutaneous intervention results.
Characteristics of the Patient Population and Procedural Variables
| Age, y | 61 ± 12 |
| Sex, male | 96 (83) |
| Arterial hypertension | 54 (47) |
| Diabetes mellitus | 26 (22) |
| Hypercholesterolemia | 50 (43) |
| Current smoker | 59 (51) |
| Pain onset to first medical contact, min | 90 [45-149] |
| First medical contact – balloon, min | 104 [84-145] |
| Total ischemia time, min | 213 [150-304] |
| Admission systolic blood pressure, mmHg | 124 ± 30 |
| Admission heart rate, bpm | 72 ± 16 |
| Admission Killip class ≥ 2 | 15 (13) |
| Radial approach | 112 (97) |
| Number of vessels with significant stenosis | |
| 1 | 67 (58) |
| 2 | 40 (35) |
| 3 | 9 (8) |
| Left main disease | 1 (0.9) |
| Culprit artery | |
| Left anterior descending | 45 (39) |
| Right coronary | 56 (48) |
| Left circumflex | 15 (13) |
| Baseline TIMI flow | |
| 0 | 113 (97) |
| 1 | 2 (2) |
| 2 | 1 (1) |
| TIMI flow after wire crossing | |
| 0 | 58 (50) |
| 1 | 37 (32) |
| 2 | 19 (16) |
| 3 | 2 (2) |
| TIMI flow after aspiration | |
| 0 | 21 (18) |
| 1 | 14 (12) |
| 2 | 58 (50) |
| 3 | 23 (20) |
| TIMI flow after procedure | |
| 0 | 1 (1) |
| 1 | 3 (3) |
| 2 | 15 (13) |
| 3 | 97 (83) |
| Baseline TIMI thrombus grade | |
| 4 | 4 (3) |
| 5 | 112 (97) |
| TIMI thrombus grade after aspiration | |
| < 3 | 62 (53) |
| ≥ 3 | 54 (47) |
| Culprit vessel diameter, mm | 3.3 ± 0.6 |
| Stent diameter, mm | 3.4 ± 0.5 |
| Total stenting length, mm | 28.7 ± 12 |
| Direct stenting | 58 (50) |
| Postdilation | 27 (23) |
| Drug-eluting stent | 68 (59) |
| Acute myocardial infarction location | |
| Anterior | 42 (36) |
| Inferior | 66 (57) |
| Lateral | 8 (7) |
| Number of leads with ST-segment elevation | 4 ± 2 |
| Baseline maximum ST-segment elevation, mm | 3.8 ± 2.2 |
| Postprocedure ST-segment resolution, % | 60 ± 31 |
| Aspirin | 115 |
| Clopidogrel | 59 (51) |
| Prasugrel | 47 (40) |
| Ticagrelor | 11 (10) |
| Abciximab | 34 (29) |
TIMI, Thrombolysis In Myocardial Infarction.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Plasma was obtained by centrifugation of the whole blood samples at 2000 × g for 10minutes at 4°C; the supernatants were then micro-centrifuged at 16 000 × g for 10minutes at 4°C to remove cell debris from the plasma. Samples were frozen and stored at −80°C until analysis. Cell-free circulating DNA was isolated from 500μl of plasma with the MagMAX Cell–Free DNA Isolation Kit (Applied Biosystems, Thermofisher, Madrid, Spain) according to the manufacturer's instructions, and were eluted in a final volume of 100μl Tris-EDTA buffer solution. To test the loss of cfDNA during the isolation procedure, the flow-through from all the wash steps was collected in a separate tube for each sample and the total amounts of cfDNA in the flow-through and the final eluate were measured by spectrometry (NanoDrop, Thermofisher, Madrid, Spain).
The proportion of human genomic cfDNA was determined by direct quantitative real-time polymerase chain reaction. Measurements were performed in triplicate using 5μl of cfDNA in a 20μl volume using the SYBR Select Master Mix (Applied Biosystems, Thermofisher, Madrid, Spain). The primers were MSTN (NM_005259.2) Forward: 5’-TTATGCTGATTGTTGCTGGTCC-3’ and Reverse: 5’-TGCATTACACAGCCCCTCTT-3’ and the following cycling parameters were used: 95°C for 10minutes, followed by 40 cycles of 95°C for 15 s and 60°C for 1 minute. The number of genomic cfDNA copies was established by comparison to a calibration curve based on known amounts of genomic DNA isolated from human haploid cells and was expressed in ng/mL of DNA.
As systemic cfDNA can show a high interindividual variability due to the wide range of conditions (physiological apoptosis) and diseases including cancer (pathological apoptosis and necrosis) that can elevate its levels,11 we used the peripheral-coronary cfDNA difference (gradient) as an indicator of the coronary production of cfDNA in an individual patient. In addition, due to the abovementioned logistic constraints of the primary coronary intervention procedure, the blood sample for peripheral cfDNA determination was drawn with little delay with respect to the coronary sample, ie, in a little more evolved phase of the infarction. This fact might induce a higher peripheral cfDNA concentration. Our assumption was that the smaller the peripheral-coronary cfDNA gradient, the greater the coronary cfDNA burden.
Additionally, intracoronary and peripheral myeloperoxidase and high-sensitivity cardiac troponin T were also measured in a subgroup of 95 patients from the University Clinic Hospital in Valencia, Spain. Myeloperoxidase was measured using a human myeloperoxidase ELISA kit (Cayman chemical, Ann Arbor, Michigan, United States) and an Elecsys assay (Roche Diagnostics, Switzerland) was used to determine troponin.
EndpointsThe primary endpoint was the lack of ST-segment resolution (STR) (≥ 70%) 60minutes following the procedure as a parameter of coronary reperfusion. The secondary endpoint was the absence of TIMI 3 flow at the end of the procedure.
Statistical AnalysisContinuous variables are expressed as the mean and standard deviation and were compared using unpaired t-tests, while categorical variables are presented as absolute numbers and percentages and were compared with chi-square tests. Cell-free DNA values had a non-Gaussian distribution according to the Kolmogorov-Smirnov test and so these values are expressed as the median with their 25% confidence interval (25%CI) to 75% confidence interval (75%CI) and compared using the Wilcoxon and Mann-Whitney U tests. Their correlation was assessed using the Spearman coefficient.
Receiver operating characteristic curves were constructed to assess the discrimination accuracy of cfDNA gradient for the endpoints. Optimal cutoff values were derived from receiver operating characteristic curves maximizing the specificity for the primary endpoint. The relationship between peripheral-coronary cfDNA gradient and the lack of STR or final TIMI 3 flow, was tested by univariate analysis. We also performed a preliminary univariate analysis to identify among all clinical, angiographic, and procedural variables (Table 1), those that were related to the endpoints (P < .10). The variables related to STR were the time from pain onset to first medical contact, age, hypertension, hypercholesterolemia, smoking, TIMI flow after wire crossing, and left circumflex culprit artery. For final TIMI 3 flow, they were Killip class ≥ 2, thrombus degree < 3 after aspiration, culprit vessel diameter and stent length. Next, a logistic regression model was built including the cfDNA gradient along with the related variables identified in the univariate analysis. The odds ratios (OR), 95%CI and C-statistics of the models were estimated. Finally, we also analyzed the relationship between cfDNA and myeloperoxidase or troponin.
RESULTSBaseline Patient CharacteristicsThe characteristics of the patient population are shown in Table 1. The mean maximum ST-segment elevation was 3.8 ± 2.2 millimeters prior to the primary percutaneous intervention and the baseline flow grade was TIMI 0 in 113 (97%) patients, TIMI 1 in 2 patients, and TIMI 2 in 1 patient. After the infarction-related lesion was crossed with the wire, 21 patients (18%) showed a TIMI flow grade ≥ 2. A mean 4.1 ± 1.7 manual aspirations were performed, and a macroscopic thrombus was obtained in 80 (69%) patients. After thrombus aspiration, 62 (53%) patients showed a minimum residual angiographic thrombus (TIMI thrombus grade < 3) and 81 (70%) had TIMI flow ≥ 2. At the end of the procedure, TIMI 3 flow was observed in 97 (83%) patients and ST resolution in 51 (44%) patients.
Cell-free DNAPeripheral and coronary cfDNA values were strongly correlated (Spearman coefficient = 0.80; P = .0001). There were no significant differences between them: peripheral cfDNA = 5.31 (25%CI to 75%CI, 3.03-11.84); coronary cfDNA = 5.27 (25%CI to 75%CI, 2.73-13.41) ng/mL; P =.57.
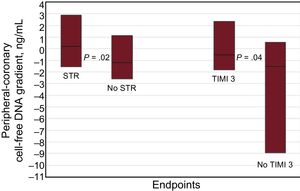
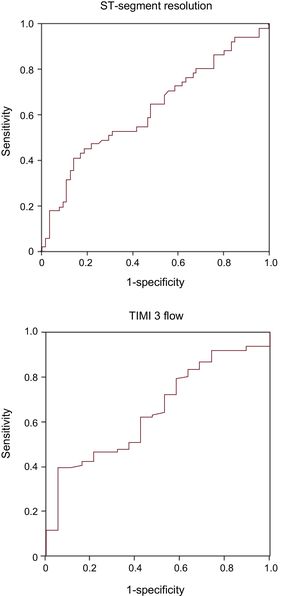
A total of 52 patients (45%) showed a positive and 64 (55%) negative peripheral-coronary cfDNA gradient. Patients without STR and final TIMI 3 flow had a smaller peripheral-coronary cfDNA gradient (P = .02 and P = .04, respectively, Figure 1). Figure 2 depicts the receiver operating characteristic curves of cfDNA gradient for STR and final TIMI 3 flow. The derived optimal cutoff value was 1.82 ng/mL, with an 86% specificity and 41% sensitivity for STR. Indeed, a small peripheral-coronary cfDNA gradient (< 1.82 ng/mL) was associated with a higher rate of no STR (65% vs 30%; OR 4.4, 95%CI, 1.8-10.7; P = .001) and lack of final TIMI 3 flow (21% vs 3%; OR, 7.7; 95%CI, 1.0-60.2; P = .05). The mean time delay between the collection of the coronary and peripheral blood samples was 21 ± 11minutes. There were no differences in the blood samples delay between the subgroups above or below the cfDNA gradient cutoff (21.0 ± 10.5 vs 20.1 ± 11.9, minutes).
Comparison of the cell-free DNA gradient in absolute values (ng/mL), between patients with and without STR, and between patients with and without TIMI 3 flow at the end of the primary percutaneous coronary intervention. The boxes represent interquartile ranges and the horizontal line in each box represents the median. STR, ST-segment resolution; TIMI, Thrombolysis In Myocardial Infarction. STR = 0.87; 25%CI to 75%CI, –1.55-2.87 ng/mL. No STR = –0.89; 25%CI to 75%CI, –257 to 1.12 ng/mL. TIMI 3 = –0.38; 25%CI to 75%CI, –1.80 to 2.32 ng/mL. No TIMI 3 = –1.08; 25%CI to 75%CI, –8.94 to 0.57 ng/mL.
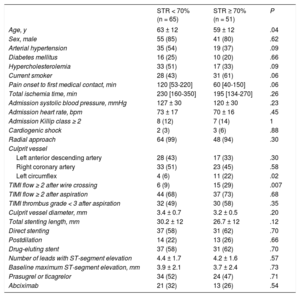
Table 2 shows the clinical, angiographic, and procedural variables that were related to STR in the univariate analysis. Compared with patients with STR, patients without STR were older, the left circumflex was less frequently the infarction-related artery, and they were less likely to have a TIMI flow ≥ 2 after wire crossing. In addition, a longer time from pain onset to the first medical contact, hypertension, hypercholesterolemia and no smoking, tended toward an association with the absence of STR. In the logistic regression analysis, a peripheral-coronary cfDNA gradient < 1.82 ng/mL was predictive of no STR (OR, 4.50; 95%CI, 1.60-12.62; P = .004), along with the involvement of left anterior descending or right coronary arteries (OR, 4.54; 95%CI, 1.17-17.63; P = .03), and initial TIMI flow < 2 after wire crossing (OR, 6.19; 95%CI, 1.86-20.57; P = .003); other variables showing a nonsignificant trend were time from pain onset to first medical contact (P = .09), hypercholesterolemia (P = .1) and smoking (P = .06). The C-statistic of the model was 0.80 (95%CI, 0.72-0.88; P = .0001). The predictive variables of not achieving TIMI 3 flow at the end of the procedure were admission Killip class ≥ 2 (OR, 9.7; 95%CI, 2.3-41.2; P = .002), culprit vessel diameter (per mm; OR, 5.1; 95%CI, 1.7-15.2; P = .003) and total stented length (per mm; OR, 1.05; 95%CI, 1.0-1.1; P = .05), while cfDNA gradient did not reach statistical significance (P = .14). The C-statistic of the model was 0.84 (95%CI, 9.74-0.94; P = .0001).
Clinical, Angiographic and Procedural Characteristics in Patients With STR < 70% and STR ≥ 70%
| STR < 70% (n = 65) | STR ≥ 70% (n = 51) | P | |
|---|---|---|---|
| Age, y | 63 ± 12 | 59 ± 12 | .04 |
| Sex, male | 55 (85) | 41 (80) | .62 |
| Arterial hypertension | 35 (54) | 19 (37) | .09 |
| Diabetes mellitus | 16 (25) | 10 (20) | .66 |
| Hypercholesterolemia | 33 (51) | 17 (33) | .09 |
| Current smoker | 28 (43) | 31 (61) | .06 |
| Pain onset to first medical contact, min | 120 [53-220] | 60 [40-150] | .06 |
| Total ischemia time, min | 230 [160-350] | 195 [134-270] | .26 |
| Admission systolic blood pressure, mmHg | 127 ± 30 | 120 ± 30 | .23 |
| Admission heart rate, bpm | 73 ± 17 | 70 ± 16 | .45 |
| Admission Killip class ≥ 2 | 8 (12) | 7 (14) | 1 |
| Cardiogenic shock | 2 (3) | 3 (6) | .88 |
| Radial approach | 64 (99) | 48 (94) | .30 |
| Culprit vessel | |||
| Left anterior descending artery | 28 (43) | 17 (33) | .30 |
| Right coronary artery | 33 (51) | 23 (45) | .58 |
| Left circumflex | 4 (6) | 11 (22) | .02 |
| TIMI flow ≥ 2 after wire crossing | 6 (9) | 15 (29) | .007 |
| TIMI flow ≥ 2 after aspiration | 44 (68) | 37 (73) | .68 |
| TIMI thrombus grade < 3 after aspiration | 32 (49) | 30 (58) | .35 |
| Culprit vessel diameter, mm | 3.4 ± 0.7 | 3.2 ± 0.5 | .20 |
| Total stenting length, mm | 30.2 ± 12 | 26.7 ± 12 | .12 |
| Direct stenting | 37 (58) | 31 (62) | .70 |
| Postdilation | 14 (22) | 13 (26) | .66 |
| Drug-eluting stent | 37 (58) | 31 (62) | .70 |
| Number of leads with ST-segment elevation | 4.4 ± 1.7 | 4.2 ± 1.6 | .57 |
| Baseline maximum ST-segment elevation, mm | 3.9 ± 2.1 | 3.7 ± 2.4 | .73 |
| Prasugrel or ticagrelor | 34 (52) | 24 (47) | .71 |
| Abciximab | 21 (32) | 13 (26) | .54 |
STR, ST-segment resolution; TIMI, Thrombolysis In Myocardial Infarction.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Troponin concentrations were much higher in the peripheral than in the coronary samples (median = 478; 25%CI to 75%CI, 138-1269 vs 89 25%CI to 75%CI, 43-204 ng/L; P = .0001). Troponin levels did not correlate with cfDNA (coronary: r = –0.13; P = .2; peripheral: r = –0.01; P = .9). The troponin gradient between the peripheral and coronary levels was similar in patients with and without STR (P = .9). The same was observed for TIMI 3 flow (P = .3).
There were no differences between peripheral and coronary myeloperoxidase levels (229, 25%CI to 75%CI, 130-309 vs 220 25%CI to 75%CI, 125-291; ng/mL; P = .3). The coronary myeloperoxidase concentration weakly correlated with coronary cfDNA (r = –0.21; P = .04), and there was no correlation between the peripheral levels (r = –0.03; P = .8). None of the myeloperoxidase measurements (peripheral, coronary or its gradient) were related to STR.
DISCUSSIONIn this study we measured the peripheral and coronary cfDNA levels in patients with STEMI that were treated with primary percutaneous intervention using manual thrombus aspiration. The main finding was that a smaller cfDNA gradient between the peripheral and coronary circulations was associated with lack of coronary reperfusion. The most plausible explanation for this result is that intracoronary cfDNA levels originate in part from hyperactivated leukocytes, and that a high intracoronary cfDNA concentration relative to the systemic concentration indicates higher local inflammatory activity at the coronary culprit lesion preventing successful reperfusion.
Cell-free DNA in ST-segment Elevation Myocardial InfarctionFew studies have evaluated cfDNA in STEMI. Two studies found a rise in the serum levels attributed to myocardial necrosis and apoptosis.7,10 The observed correlation with troponin levels seems to confirm this hypothesis.8,10 In contrast, we did not find a correlation between cfDNA and troponin. Peripheral troponin levels were much higher than their coronary counterpart. This must reflect acute MI time course since the peripheral blood samples were collected with an average time delay of 21minutes. Cell-free DNA, however, did not follow the same pattern: there were no differences between peripheral and coronary levels. It is possible that the cfDNA release kinetics is slower than that of troponin. Indeed, some studies have observed a correlation between troponin and cfDNA only in later stages of MI.7,9 Still, we cannot rule out that the delay had influenced the peripheral cfDNA levels, although there were no significant differences in the time delay between patients with high or low cfDNA gradient.
The activation of neutrophils with the release of their nuclear content at the complicated coronary atherosclerotic plaque might also determine circulating cfDNA levels. Those patients with a small cfDNA gradient between the peripheral and coronary circulation had a lower likelihood of STR, even after full adjustment for well-known clinical and procedural predictors. Our hypothesis is that this finding reflects the higher degree of intracoronary inflammation.
Leukocyte Activation in ST-segment Elevation Myocardial InfarctionLeukocytes are strongly activated during STEMI and are directly involved in the process of local plaque complication.12–17 An increase in leukocyte-platelet aggregates at the culprit lesion has been observed in intracoronary blood aspirates.18,19 The main mechanism for this phenomenon is the release of chromatin fibers from neutrophils into the extracellular space; these form NETs that act as a scaffold for platelet aggregation and thrombosis.2 Intracoronary NETs were detected in the very early phase of MI and related to microvascular thrombosis.3,4 Cell-free DNA is the major structural component of NETs and its level is strongly correlated with the NETs intracoronary burden.4,20
Myeloperoxidase is a granule protein released by activated leukocytes, which catalyzes the formation of reactive species that mediate atherosclerosis plaque growth and rupture.21 We did not find a correlation between myeloperoxidase and cfDNA levels. This result is in line with a previous study.10 The explanation could be that myeloperoxidase levels are not mainly NETs-derived.
Clinical ImplicationsIn a landmark study, Ito et al.22 demonstrated that restoration of normal epicardial coronary blood flow does not guarantee myocardial reperfusion in STEMI. ST-segment resolution is a simple and immediate surrogate of myocardial reperfusion.23 Several strategies have been proposed to prevent and treat microvascular damage, though none of them have proven to be fully effective.1,24,25 Thrombus aspiration was a promising tool but large randomized trials failed to confirm its efficacy.5,6 Our study suggests that cfDNA may play a role in failed reperfusion after thrombus aspiration. We hypothesize that this is because the complexes resulting from the interaction between NETs platelets, and fibrin facilitate microembolization and make the substrate difficult to aspirate when using a simple catheter technique. Whether concomitant DNase treatment in this context might improve thrombus aspiration effectiveness warrants further investigation.
Strengths and LimitationsThe strengths of this study are the larger sample size than those of previous studies, the analysis of the correlation between cfDNA and troponin or myeloperoxidase, and the adjustment for well-known clinical and procedural predictors.
The limitations are the following: a) the 44% STR rate is lower than in other studies. This is explained by the requirement that all patients had to have a high thrombus burden in the initial angiogram (TIMI thrombus degree 4 or 5) to be included; b) as stated before, coronary and peripheral blood samples were not taken simultaneously. One might speculate that the relative higher peripheral cfDNA to the coronary levels in patients with successful reperfusion would be the consequence of the washout of the intracellular content of myocardial cells since the intracoronary blood was drawn before opening the coronary artery while the peripheral blood was obtained immediately afterwards. However, it seems that the 21-minute delay was too short to meaningfully modify cfDNA levels. It has been suggested that the kinetics of cfDNA release produced by cardiomyocyte necrosis is a slow process.7,9 Furthermore, troponin concentration, which is the purest parameter of myocardial injury, did not correlate with cfDNA. Still, we cannot rule out the possibility that the inflammatory activity linked to the reperfusion process had affected the peripheral cfDNA levels; c) flushing of the aspiration catheter before thrombectomy could have interfered with the measurement of coronary cfDNA levels; and d) the moderate sample size limits the conclusions of the study.
CONCLUSIONSHigh intracoronary cfDNA relative to peripheral levels is associated with lack of STR in STEMI. This finding may reflect neutrophil activation with production of NETS. Since all patients were treated with thrombus aspiration, it is possible to speculate that this phenomenon might contribute to thrombus aspiration failure. Additional studies are mandatory to examine neutrophil activation and NET production as a potential new therapeutic target.
FUNDINGThis work was supported by grants from Spain's Ministry of Economy and Competitiveness through the Carlos III Health Institute: RD12/0042/0010 and CB16/11/00420; ERDF (European Regional Development Fund); Health Research Fund.
CONFLICTS OF INTERESTJ. Núñez has received personal fees from Novartis, Abbott, and Vifor Pharma, outside this work.
- –
Elevation of circulating cfDNA in STEMI has been attributed to myocardial necrosis and apoptosis.
- –
Hyperactivation of neutrophils forming extracellular traps at the culprit coronary lesion might play a role in failed reperfusion in STEMI.
- –
Cell-free DNA is a major structural component of NETs.
- –
A high coronary cfDNA burden relative to peripheral cell-free DNA levels is associated with lack of STR after primary coronary intervention using thrombus aspiration.
- –
A high coronary cfDNA burden would be the result of a higher degree of inflammation at the intracoronary culprit site preventing coronary reperfusion.
- –
Neutrophil activation with production of NETs might contribute to thrombus aspiration failure in STEMI treated with primary coronary intervention.
.