Keywords
INTRODUCTION
Percutaneous implantation of an aortic valve prosthesis is considered an alternative for the treatment of severe symptomatic aortic valve stenosis with a high surgical risk, owing to its high success rate during implantation (around 95%) and its low associated hospital mortality (less than 10% in the early series1-4). Nevertheless, reports exist of a high incidence of electrocardiographic abnormalities and the need for a pacemaker due to advanced atrioventricular (AV) block with the percutaneous implantation of the CoreValve® aortic valve prosthesis (Medtronic CV, Luxemburg), reaching 33% in some series,5 as opposed to what occurs after valve replacement surgery.6,7 Little is still known about the causes, behavior, and course of new onset electrocardiographic disorders following the percutaneous implantation of an aortic valve prosthesis. Although the clinical practice guidelines recommend implanting pacemakers in patients who have persistent irreversible changes in their cardiac stimulation and conduction generating systems, the pathophysiology involved in the electrical conduction system of patients treated with the percutaneous implantation of an aortic valve prosthesis remains poorly understood. A few authors have recently examined the various possible factors associated with early and persistent conduction abnormalities after implantation of an aortic valve prosthesis.8 In addition, the need has arisen for models that can detect those patients with a higher risk of requiring a pacemaker.
The aim of this study, therefore, was to assess the incidence of electrocardiographic abnormalities and analyze the possible predictors of the need for a pacemaker due to advanced AV block following the percutaneous implantation of a CoreValve® aortic valve prosthesis.
METHODS
In 2008 our center initiated a program of evaluation and percutaneous implantation of a CoreValve® aortic valve prosthesis in patients with a high surgical risk and/or advanced age who refused valve replacement surgery. All the patients were evaluated by a multidisciplinary team, composed of surgeons, and clinical and interventional cardiologists. The selection process for the candidate patients to receive treatment with this new technique followed the consensus recommendations drawn up jointly by several scientific societies,9 as well as complying with the anatomical criteria required for the percutaneous implantation of a CoreValve® aortic valve prosthesis.3,4
During the period April 2008 to October 2009, 65 patients with severe symptomatic aortic valve stenosis and a high surgical risk were treated with the percutaneous CoreValve® aortic valve prosthesis. Of these 65 patients initially included in the study, 3 were excluded from the final analysis of the predicting factors as they had permanent pacemakers due to advanced AV block and 1 patient was excluded because he died during the procedure prior to implanting the prosthesis.
At the start, the patients were evaluated clinically, their surgical risk was estimated from the logistic EuroSCORE, and they underwent transthoracic echocardiography, coronary angiography, and an aortogram to evaluate the geometry of the aortic root and aorta-iliac-femoral axis.
Description of the Device
The CoreValve#R aortic valve prosthesis is a biological triple valve prosthesis made of porcine pericardium, fitted and sutured on top of a self-expanding nitinol structure. Two valve sizes are currently available: a small prosthesis, with the portion inserted into the native annulus measuring 26 mm, and a large prosthesis, which measures 29 mm. The length of both prosthesis is 50 mm.
Procedure
The patients take 100 mg of acetylsalicylic acid prior to the procedure and continue taking it indefinitely. As well as receiving a loading dose of 300 mg of clopidogrel, they continue taking 75 mg of clopidogrel for at least 6 months. During the procedure, sodium heparin was administered intravenously, adjusting the dose for weight (0.5 mg/kg). Antibiotic prophylaxis was given with cephalosporins, or vancomycin if the patient was allergic to betalactams.
All the procedures were carried out under local anesthesia with superficial sedation. In most cases access was via the femoral artery, with an 18F introducer, and the femoral puncture closed with the Prostar XL 10 Fr percutaneous closure device (Abbot Vascular Devices, Redwood City, California).
In 3 patients the left subclavian artery was used for the approach, in collaboration with the cardiac surgeon who performed the opening and closure of the artery.
After placing a transitory pacemaker catheter via the right jugular vein, the femoral artery was punctured and used for valve implantation, after which the vascular access device was left in place. Aortic valvuloplasty was then done with cardiac overstimulation at a frequency of between 150 and 180 beats per minute in order to prevent displacement of the balloon. Following this the aortic prosthesis was released retrogressively, under fluoroscopic and angiographic guidance. After the procedure the patients were monitored by telemetry for 4 days and by a control echocardiogram after 72 h.
The procedure was considered to be a success if the implantation was correct, the prosthesis functioned normally, and the patient survived the procedure.
Electrocardiographic Study
All the patients underwent an electrocardiogram (ECG) before and after the percutaneous aortic valve implantation. Data were recorded on the rhythm, heart rate (independently of the rhythm) and the PR, QT and QRS complex intervals (measured in milliseconds at a speed of 25 mm/s), as well the presence of bundle branch block (complete and incomplete) or advanced AV block in accordance with the criteria recommended by the World Health Organization and the International Society and Federation for Cardiology Task Force to define hemiblock, right bundle branch block (RBBB), and left bundle branch block (LBBB).10 Implantation of a permanent pacemaker was indicated in the presence of third or second degree AV block (Mobitz II), following the recommendations of the Spanish Society of Cardiology for patients with acquired AV block in special situations such as valve surgery.11
During the procedure all the patients were monitored electrocardiographically with 3-lead recordings, and a transitory pacemaker was inserted via the jugular vein in order to perform cardiac overstimulation during the aortic valvuloplasty prior to implanting the prosthesis and as prevention in the event of advanced AV block after the implant.
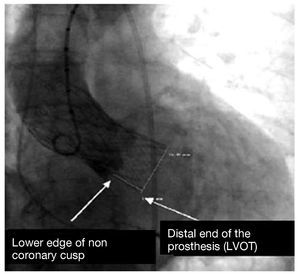
Quantitative angiography was used to measure the depth of the prosthetic structure in the outflow tract, taken as the distance (in millimeters) from the noncoronary cusp to the distal end of the prosthesis situated in the left ventricle, as described by Piazza et al8 (Figure 1). The cranial left oblique view, commonly chosen to perform the release and implant of the prosthesis, was calculated to determine the degree of periprosthetic regurgitation. All the studies were evaluated independently by two cardiologists. The interpretation of the images was done by readers who were unaware of the clinical situation. The inter-observer degree of agreement, assessed using the intraclass correlation coefficient, was found to be 0.9 (95% confidence interval [CI], 0.85-0.94; P<.001); the mean was used as the distance to use.
Figure 1. Angiographic quantification of the depth of the prosthesis in the left ventricular outflow tract (LVOT). Taken from Piazza et al.8
Follow-up
All the patients underwent clinical follow-up in the hemodynamics office of our center. They were all evaluated after 1, 3, 6, and 12 months and then every 6 months after the aortic prosthesis implant. At each visit a 12-lead surface ECG was done and the pacemakers in those patients who had one were checked.
Statistical Analysis
The values of the continuous variables are expressed as the mean (standard deviation) and those of the categorical variables as percentages. The χ2 test was used for the qualitative variables, and the continuous variables were assessed with the Student t test for paired data, as the variables all had a normal distribution. The multivariate analysis for the prediction of the need for a pacemaker was done using Cox regression, and included all the variables that were significant in the univariate analysis as well as those that were considered necessary to adjust the model correctly. The results are given with the odds ratio (OR) and the 95% CI. To evaluate the role of the depth of the aortic prosthesis in the left ventricular outflow tract (LVOT), the sensitivity and specificity were calculated and the cut point through the receiver operating characteristic (ROC) curve was identified. The degree of concordance between the two observers was obtained by calculating the intraclass correlation coefficient. Statistical significance was set at P<.05. The data were analyzed with SPSS software, version 12.0 (Chicago, Illinois, United States).
RESULTS
Baseline and Electrical Characteristics Prior to the Procedure
The baseline characteristics are summarized in Table 1. The mean age of the patients was 79 (7.8) years and the mean EuroSCORE was 20 (14%). Before the implant procedure, 27.8% of the patients were in atrial fibrillation and 44 patients (72.3%) were in sinus rhythm. Three patients had a pacemaker in VVI mode due to advanced AV block.
Electrocardiographic abnormalities (hemiblock, RBBB, or LBBB) were present in 82% of the patients. The baseline heart rate was 72 (13) (48-107) beats per minute.
Electrocardiographic Abnormalities After the Implant
After implanting the aortic prosthesis, 21 of the 61 patients required a permanent pacemaker due to advanced AV block (with advanced considered to be second or third degree AV block); this corresponds to 34.4% of the patients, after excluding from the analysis the 3 patients who already had pacemakers and the patient who died before the prosthesis was implanted. Permanent pacemaker implanted between 2 and 5 days after the procedure. Of the patients who required a permanent pacemaker, 81% had advanced AV block just after implanting the prosthesis and 4 patients had it between the third and fourth day.
The mean duration of the PR and QRS intervals increased from 165 (52) to 185 (59) ms (P=.017) and 87.6 (34) to 136 (34) ms (P<.001), respectively (Table 2). One of the patients experienced paroxysmal atrial fibrillation before the procedure, but which ceased after the implantation.
Predictors of a Permanent Pacemaker
The need for a permanent pacemaker was associated with a greater depth of prosthetic implantation in the LVOT, with a mean distance of 13 (2.5) mm in the patients who required a permanent pacemaker as opposed to 8.8 (2.8) mm in those who did not require one (P<.001). No differences were found concerning the dimensions of the aortic annulus or the prosthesis/annulus ratio, the degree of left ventricular hypertrophy or valve calcification. The patients with RBBB required a pacemaker more often than those who had LBBB (Table 3).
Furthermore, the depth of the prosthesis in the LVOT was the only predictor of the need for a pacemaker in the multivariate analysis (OR=1.9; 95% CI, 1.19-3.05; P=.007) (Table 4).
Sensitivity and Specificity of the Prosthesis Depth in the Left Ventricular Outflow Tract
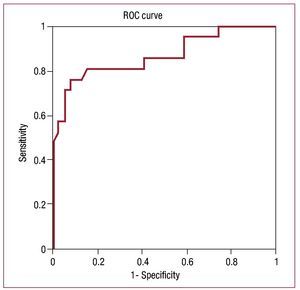
To determine the cut point, we used the ROC curve (Figure 2). A prosthesis depth of 11.1 mm in the LVOT had a sensitivity of 81% and a specificity of 84.6%, with an area under the curve of 0.86 (0.75-0.96), to predict the need for a definitive pacemaker due to advanced AV block. The positive and negative predictive values were 74% and 89.4%, respectively, with a diagnostic precision of 83.6%.
Figure 2. Receiver operating characteristic (ROC) curve. Depth of the aortic prosthesis in the left ventricular outflow tract. Area under the curve, 0.86 (0.75-0.96).
Follow-up
On discharge, 29 patients (47.5%) had LBBB, 21 (34.4%) had a pacemaker rhythm, and the remainder lacked any important conduction abnormalities.
After a mean follow-up of 7.1 (5) months, 6 (9.2%) patients had died. One of these patients experienced sudden death 3 months after the procedure. There was no worsening or progression of the electrical abnormalities during the follow-up (the same number of patients still had LBBB), except for the sudden death. It was not necessary to implant any further pacemakers and 3 patients who had received a pacemaker recovered their own rhythm, without requiring stimulation, 3-6 months after the implant procedure. The percentage stimulation of the other patients who had a pacemaker was greater than 90%.
DISCUSSION
In our series a permanent pacemaker was required after the prosthetic valve implant procedure by 34.4% of the patients. This figure is similar to that found by Grube et al5 but higher than various others; for example, the 9.3% reported in a registry of 646 patients who received a CoreValve® prosthetic valve,12 or the 6% to 6.5% in several surgical series,6,7,13 as well as the 4.4% to 5.4% reported with the percutaneous implantation of the EdwardsSAPIEN prosthesis.14
This high implant rate may be due to the fact that it is often performed for prophylactic reasons, as we are unaware of the evolution of new onset conduction disorders, such as bradycardia or LBBB, and their prognostic impact. In our series, one patient who had LBBB (QRS width, 180 ms) after the valve implant procedure died suddenly during the follow-up. On the other hand, 3 patients who received a pacemaker because they had advanced AV block during the first 24-48 h after the procedure recovered their own rhythm between 3 and 6 months after the implant. After the procedure, 47.5% of the patients in our series had LBBB, which remained during the medium-term follow-up.
The close anatomical relationship between the complex aortic valve structure and the intertwining between the AV node and the exit of the bundle of His (in the diagram shown by Tawara,15-17 the left branch is situated about 2-3 mm below the triangle separating the noncoronary valve and the right coronary valve) could provide an explanation for the increase in electrical conduction abnormalities that occur after the release and for the impact of the aortic prosthesis on these structures.18 In addition, a high percentage of patients have some sort of conduction disorder prior to the procedure.
The greater surface area of the prosthesis in contact with the LVOT and the characteristics of nitinol, which provides persistent self-expansion, could account for the differences in the percentage of pacemaker implants between the two percutaneous valve types.8,19 When the bioprosthesis and the adjacent cardiac structures have adapted to the tensional stress to which they are submitted, the irritative effect in the conduction system may be reduced, leading to re-establishment of the heart's own electrical conduction. This could explain the recovery of the natural heart rhythm seen in some patients in our series. In a necropsy after the implantation of an Edwards-SAPIEN prosthesis, Moreno et al20 found the histological presence of a hematoma at the interventricular septum that compromised the bundle of His, which would explain the changes seen in the AV conduction system.
In our study we noted that the depth of the prosthetic structure in the LVOT was greater in those patients who required a permanent pacemaker due to advanced AV block (13 [2.5] vs 8.8 [2.8] mm; P<.001). Furthermore, a depth of ≥11.1 mm could be an early indicator of the need for a pacemaker, with high degrees of sensitivity and specificity. These data suggest that a higher position of the aortic prosthesis might reduce the incidence of electrical changes and the need for a pacemaker. In fact, implant of the CoreValve® prosthetic valve is considered optimal when the depth of the prosthesis in the LVOT is 6 mm. Further specific studies are required to examine these aspects and determine the indications for the implant of a permanent pacemaker in these patients.
Several studies have recently shown that pacemaker need can be predicted from the combination of a few variables, such as the presence of LBBB with left axis deviation, an interventricular septal thickness >17 mm or noncoronary cusp thickness >8 mm, with 75% sensitivity and 100% specificity.21 This predictive model, presented by Jilaihawi et al,21 should be validated in series with larger numbers of patients and, if confirmed, could be used to select candidates for the preventive implant of a pacemaker and hasten recovery after the procedure. For patients with a positive index in this predictive model, a strategy of preventive pacemaker implant and early discharge could be applied, whereas those patients with a negative index would prolong their hospital stay for at least 6 days after the procedure, with the aim of detecting any late conduction disorders. Baan et al22 analyzed the results of 30 patients and concluded that the presence of a small LVOT, calcification of the mitral annulus, and the prior presence of conduction disorders were associated with the need for a pacemaker, though the depth of the prosthesis was only related with the presence of new onset LBBB.
In our series, 81% of the patients who required a permanent pacemaker experienced AV block during the procedure, and 4 patients developed it between the third and fourth post-procedure days. Implant of a permanent pacemaker was mostly performed between the second and fifth day after the procedure, with no delay in discharge, though there was one sudden death 3 months after the procedure. Thus, these predictive models are necessary to prevent events secondary to late conduction disorders.
Special consideration must be given to those patients with RBBB before the procedure, since 47.6% of these required a permanent pacemaker. Although this was associated with pacemaker need in our series, it was not found to be a predictor in the regression model, possibly because of the sample size. Nevertheless, given the prevalence of new onset LBBB, it is necessary to control and evaluate pacemaker need in these patients.
Accordingly, new indications for cardiac stimulation are appearing that will require study and incorporation into the next set of clinical practice guidelines.
This study has a few limitations. As it was a single-center study with a small number of patients receiving the CoreValve® aortic prosthesis, the results cannot readily be generalized. The reproducibility of the quantitative methods should be validated in other series with greater numbers of patients, although our data do agree with those from initial reports and the observer variability was minimized by performing angiographic quantification by two independent observers. In addition, the requirement has arisen to determine the most suitable imaging technique to quantify the depth of the aortic valve prosthesis in the LVOT and avoid certain difficulties related to angiography in viewing the noncoronary cusp.
CONCLUSIONS
In our series, after implant of the CoreValve® aortic valve prosthesis, a high percentage of patients required a permanent pacemaker due to advanced AV block. The only independent predictor was the depth of the prosthesis in the LVOT, which could be used as an early indicator of the need for a pacemaker.
ABBREVIATIONS
AV: atrioventricular
ECG: electrocardiogram
LBBB: left bundle branch block
LVOT: left ventricular outflow tract
RBBB: right bundle branch block
ROC: receiver operating characteristic
Correspondence: Dr. A.J. Muñoz García.
Servicio de Cardiología. HCU Virgen de la Victoria. Campus de Teatinos, s/n. 29010 Malaga. Spain.
E-mail: ajmunozgarcia@secardiologia.es
Received March 16, 2010.
Accepted for publication July 15, 2010.