Circulating galectin-3 (Gal-3) is elevated and significantly correlates with all-cause and cardiovascular mortality in patients with heart failure. However, the relationship between serum Gal-3 and heart transplant (HT) outcomes is unclear. The aim of this study was to describe the longitudinal trend and prognostic value of Gal-3 levels after HT.
MethodsBanked serum samples were available from 122 HT recipients, collected before transplant and at 1, 3, 6, and 12 months posttransplant. Gal-3 levels in these serum samples were measured by enzyme immune assay. Multivariable Cox regression was performed to determine the prognostic value of 12-month posttransplant Gal-3 serum levels. The primary endpoint was the composite variable all-cause death or graft failure over long-term posttransplant follow-up.
ResultsCirculating Gal-3 concentration steadily decreased during the first year after HT (median values: pretransplant, 19.1 ng/mL; 1-year posttransplant, 14.6 ng/mL; P<.001). Circulating Gal-3 levels 1-year posttransplant were associated with an increased risk of all-cause death or graft failure (adjusted HR per 1 ng/mL, 1.04; 95%CI, 1.01-1.08; P=.008). The predictive accuracy of this biomarker was moderate: area under the ROC curve, 0.72 (95%CI, 0.60-0.82; P<.001).
ConclusionsCirculating Gal-3 steadily decreased during the first year after HT. However, 1-year posttransplant Gal-3 serum levels that remained elevated were associated with increased long-term risk of death and graft failure.
Keywords
The protein galectin-3 (Gal-3) is part of a family of lectins involved in inflammation and fibrosis in relation to the heart and vascular system,1 as well as other organs, including the kidneys, liver, and pancreas.2 Clinical interest in serum Gal-3 has recently increased because it shows potential as a useful biomarker in patients with cardiovascular disease.3 Patients with heart failure (HF) show particularly high Gal-3 serum levels,4 which are significantly correlated with all-cause mortality and cardiovascular mortality in this population.5
Heart transplant (HT) is the therapy of choice for patients with advanced HF, offering excellent postoperative survival and quality of life.6 Chronic inflammation and interstitial fibrosis,7 together with ischemia, cellular hypertrophy and increased wall stiffness, are major determinants of long-term myocardial remodeling of the transplanted heart graft. The association of serum Gal-3 with the first 2 processes supports the exploration of the potential clinical usefulness of this biomarker in heart HT recipients.
In a single-center study, Coromilas et al.8 reported significantly decreased Gal-3 serum levels following HT, and noted that persistently elevated serum Gal-3 levels after HT correlated with a higher occurrence of coronary allograft vasculopathy (CAV). In contrast, Grupper et al.9 found that Gal-3 serum levels did not significantly change after HT, nor were they significantly correlated with the intensity of myocardial Gal-3 expression, the grade of cardiomyocyte hypertrophy, or fibrosis of the graft. Rather, these authors stated that Gal-3 serum levels were strongly influenced by renal function. Franeková et al.10 observed a significant correlation between Gal-3 serum levels, as assessed 10 days after HT, and reduced left ventricular ejection fraction 1 year after the intervention; however, no association between this biomarker and survival was found in this study.
The aim of our study was to analyze the trend of Gal-3 serum levels over the first year after HT, and to explore its potential prognostic value as a biomarker in HT recipients.
METHODSStudy PopulationThis study included 122 adult patients who survived at least 1 year after undergoing orthotopic HT at our institution between 2004 and 2014. Banked serum samples were available from these patients. These were obtained following a prospective protocol when the patient was added to the HT waiting list, and during clinical visits or elective hospital admissions at 1, 3, 6, and 12 months after HT. Gal-3 serum levels were determined from these serum samples. All study participants gave their written informed consent, and the study protocol was approved by the local committee for ethics in clinical investigation.
Sample Extraction and ProcessingSerum samples were obtained by centrifugation of peripheral blood samples for 15minutes at 3000 revolutions per minute. Once serum was separated from the cellular fraction, it was divided into 0.5-mL aliquots and stored at –80°C.
Gal-3 serum levels were measured by ELISA (enzyme-linked immunosorbent assay) of Gal-3 (BMG Lab-Technologies, Offenburg, Germany). Abnormally high Gal-3 serum levels were defined by a cutoff value of ≥ 17.8 ng/mL, based on previous studies.8
Data CollectionClinical data were extracted from a prospectively maintained database, and completed through individualized retrospective review of the clinical records of all study participants. We recorded relevant clinical variables of the HT recipients from before HT and for up to 1 year after the intervention, as well as long-term clinical outcomes. Information about patients’ vital signs and episodes of hospital admission as of August 31, 2016 was available from our database.
Clinical OutcomesThe major clinical outcome of this study was the combined variable “all-cause death or hospitalization due to graft failure” over a long-term follow-up beyond 1 year after HT. We also separately analyzed both components of this major composite outcome. Graft failure was defined as the first hospitalization due to typical symptoms of HF, together with objective evidence of substantial abnormality of graft structure or function, such as reduced left ventricular ejection fraction, restrictive physiology, or right ventricular dysfunction, proposed as causing the clinical picture.
At 1 year after HT, coronary angiography was performed to determine the presence of CAV, defined as a grade of ≥ 1 according to the current guidelines of the International Society of Heart and Lung Transplantation.11 Endomyocardial biopsies were also performed at 1 year post-HT to determine the presence of acute cellular rejection, defined by a grade ≥ 1R according to the International Society of Heart and Lung Transplantation consensus definition.12
Statistical AnalysisCategorical variables are presented as proportions, while continuous variables are shown as mean±standard deviation, or median and interquartile range (IQR), depending on their distribution. For all comparisons, a P value of<.05 was considered to indicate significance. Statistical analyses were performed using SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, New York, United States).
To analyze the trend of Gal-3 serum levels over the first year after HT, we used ANOVA (analysis of variance) for repeated measurements, corrected with the Greenhouse-Geisser method. We also used the Wilcoxon test for paired samples to compare the pretransplant Gal-3 serum levels with those at 1, 3, 6, and 12 months after HT.
Applying backward stepwise linear regression, we performed an exploratory analysis to identify clinical factors associated with Gal-3 serum levels at 1 year after HT. In the first step of backward stepwise linear regression analysis, we entered candidate variables that reflected the recipients’ clinical characteristics before HT and at 1-year posttransplant, and determined which showed a univariable association with 1-year posttransplant Gal-3 serum levels with a P value of<.010.
We used Cox regression to assess the potential association of Gal-3 serum levels at 1 year post-HT with long-term prognosis beyond this time point. Patients included in this analysis were followed up for a maximum of 10 years. Multivariable adjustment was performed to control potential confusion bias that might affect the validity of statistical associations detected between posttransplant Gal-3 serum levels and long-term outcomes. We constructed a backward stepwise multivariable Cox regression model, including in the initial step several covariables that were considered potential confounders based on clinical experience, previous reports, and/or a statistically significant association with posttransplant serum Gal-3 levels in our cohort. These covariables were recipient age, donor age, recipient sex, donor sex, left ventricular ejection fraction, right atrial pressure, glomerular filtration rate, tacrolimus use, and mycophenolate mofetil use.
The defined multivariable model was used to estimate the adjusted hazard ratio (HR) for the major clinical outcome of the study (all-cause death or graft failure) in patients with abnormally high Gal-3 serum levels (≥ 17.8 ng/mL) at 1 year posttransplant compared with the rest of the cohort. We used the Kaplan-Meier method to estimate “cumulative survival free of death or admission due to graft failure” for both patient groups, and used the log-rank test for between-group comparisons.
The accuracy of 1-year posttransplant Gal-3 serum levels in predicting the occurrence of the major clinical outcome of the study and its 2 individual components over long-term follow-up was assessed by means of ROC (receiver operating characteristic) curves. The area under the ROC curve was assessed by means of a nonparametric approach.
RESULTSPatientsFrom 2004 to 2014, 238 patients aged ≥ 18 years underwent HT in our institution, among which 202 (84.9%) survived at least 1 year after the intervention. Of these participants, 122 were enrolled in the present study.
Banked serum samples from before HT and at 1, 3, 6, and 12 months after HT were available from 105, 102, 102, 100, and 99 patients, respectively. Complete sets of consecutive measurements were available from 53 patients.
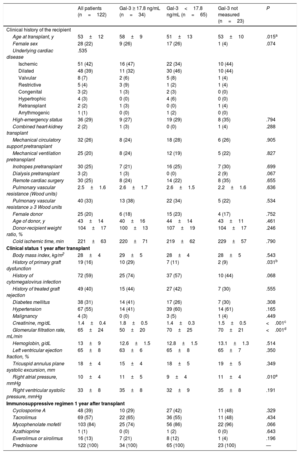
Table 1 shows the patients’ clinical characteristics before HT and at the routine clinical visit at 1 year after transplant, both in the whole study population and also in the subgroups of patients with elevated (≥ 17.8 ng/mL), normal (< 17.8 ng/mL) and unknown (not measured) Gal-3 serum levels, 1 year after transplant.
Comparison of Clinical Characteristics of Patients With Elevated (≥ 17.8 ng/mL), Normal (< 17.8 ng/mL) or Unknown (not Measured) Gal-3 Serum Levels at 1 Year After Heart Transplant
| All patients (n=122) | Gal-3 ≥ 17.8 ng/mL (n=34) | Gal-3<17.8 ng/mL (n=65) | Gal-3 not measured (n=23) | P | |
|---|---|---|---|---|---|
| Clinical history of the recipient | |||||
| Age at transplant, y | 53±12 | 58±9 | 51±13 | 53±10 | .015a |
| Female sex | 28 (22) | 9 (26) | 17 (26) | 1 (4) | .074 |
| Underlying cardiac disease | .535 | ||||
| Ischemic | 51 (42) | 16 (47) | 22 (34) | 10 (44) | |
| Dilated | 48 (39) | 11 (32) | 30 (46) | 10 (44) | |
| Valvular | 8 (7) | 2 (6) | 5 (8) | 1 (4) | |
| Restrictive | 5 (4) | 3 (9) | 1 (2) | 1 (4) | |
| Congenital | 3 (2) | 1 (3) | 2 (3) | 0 (0) | |
| Hypertrophic | 4 (3) | 0 (0) | 4 (6) | 0 (0) | |
| Retransplant | 2 (2) | 1 (3) | 0 (0) | 1 (4) | |
| Arrythmogenic | 1 (1) | 0 (0) | 1 (2) | 0 (0) | |
| High-emergency status | 36 (29) | 9 (27) | 19 (29) | 8 (35) | .794 |
| Combined heart-kidney transplant | 2 (2) | 1 (3) | 0 (0) | 1 (4) | .288 |
| Mechanical circulatory support pretransplant | 32 (26) | 8 (24) | 18 (28) | 6 (26) | .905 |
| Mechanical ventilation pretransplant | 25 (20) | 8 (24) | 12 (19) | 5 (22) | .827 |
| Inotropes pretransplant | 30 (25) | 7 (21) | 16 (25) | 7 (30) | .699 |
| Dialysis pretransplant | 3 (2) | 1 (3) | 0 (0) | 2 (9) | .067 |
| Remote cardiac surgery | 30 (25) | 8 (24) | 14 (22) | 8 (35) | .655 |
| Pulmonary vascular resistance (Wood units) | 2.5±1.6 | 2.6±1.7 | 2.6±1.5 | 2.2±1.6 | .636 |
| Pulmonary vascular resistance ≥ 3 Wood units | 40 (33) | 13 (38) | 22 (34) | 5 (22) | .534 |
| Female donor | 25 (20) | 6 (18) | 15 (23) | 4 (17) | .752 |
| Age of donor, y | 43±14 | 40±16 | 44±14 | 43±11 | .461 |
| Donor-recipient weight ratio, % | 104±17 | 100±13 | 107±19 | 104±17 | .246 |
| Cold ischemic time, min | 221±63 | 220±71 | 219±62 | 229±57 | .790 |
| Clinical status 1 year after transplant | |||||
| Body mass index, kg/m2 | 28±4 | 29±5 | 28±4 | 28±5 | .543 |
| History of primary graft dysfunction | 19 (16) | 10 (29) | 7 (11) | 2 (9) | .031b |
| History of cytomegalovirus infection | 72 (59) | 25 (74) | 37 (57) | 10 (44) | .068 |
| History of treated graft rejection | 49 (40) | 15 (44) | 27 (42) | 7 (30) | .555 |
| Diabetes mellitus | 38 (31) | 14 (41) | 17 (26) | 7 (30) | .308 |
| Hypertension | 67 (55) | 14 (41) | 39 (60) | 14 (61) | .165 |
| Malignancy | 4 (3) | 0 (0) | 3 (5) | 1 (4) | .449 |
| Creatinine, mg/dL | 1.4±0.4 | 1.8±0.5 | 1.4±0.3 | 1.5±0.5 | <.001c |
| Glomerular filtration rate, mL/min | 65±24 | 50±20 | 70±25 | 70±21 | <.001d |
| Hemoglobin, g/dL | 13±9 | 12.6±1.5 | 12.8±1.5 | 13.1±1.3 | .514 |
| Left ventricular ejection fraction, % | 65±8 | 63±6 | 65±8 | 65±7 | .350 |
| Tricuspid annulus plane systolic excursion, mm | 18±4 | 15±4 | 18±5 | 19±5 | .349 |
| Right atrial pressure, mmHg | 10±4 | 11±5 | 9±4 | 11±4 | .010e |
| Right ventricular systolic pressure, mmHg | 33±8 | 35±8 | 32±9 | 35±8 | .191 |
| Immunosuppressive regimen 1 year after transplant | |||||
| Cyclosporine A | 48 (39) | 10 (29) | 27 (42) | 11 (48) | .329 |
| Tacrolimus | 69 (57) | 22 (65) | 36 (55) | 11 (48) | .434 |
| Mycophenolate mofetil | 103 (84) | 25 (74) | 56 (86) | 22 (96) | .066 |
| Azathioprine | 1 (1) | 0 (0) | 1 (2) | 0 (0) | .643 |
| Everolimus or sirolimus | 16 (13) | 7 (21) | 8 (12) | 1 (4) | .196 |
| Prednisone | 122 (100) | 34 (100) | 65 (100) | 23 (100) | — |
Gal-3, galectin-3.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
Statistical comparisons among subgroups:
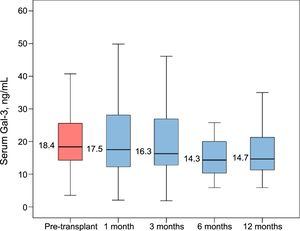
ANOVA for repeated measurements revealed a statistically significant tendency of declining serum Gal-3 levels over the first posttransplant year (P for linear trend=.030; Figure 1).
Pretransplant Gal-3 serum levels were significantly higher than both 6-month post-HT levels (P=.002) and 1-year post-HT levels (P=.001). However, pretransplant Gal-3 serum levels did not significantly differ from 1-month post-HT levels (P=.787) or 3-month post-HT levels (P=.750).
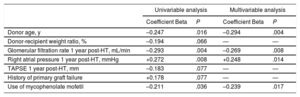
Clinical Factors Related to Gal-3 Serum LevelsTable 2 presents the results of univariable and multivariable linear regression analyses designed to identify clinical factors associated with 1-year posttransplant Gal-3 serum levels. The results showed that 1-year posttransplant Gal-3 serum levels were directly correlated with right atrial pressure and inversely correlated with donor age, mycophenolate mofetil use, and glomerular filtration rates at 1 year post-HT.
Clinical Variables Associated With Gal-3 Serum Levels at 1 Year Posttransplant
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Coefficient Beta | P | Coefficient Beta | P | |
| Donor age, y | –0.247 | .016 | –0.294 | .004 |
| Donor-recipient weight ratio, % | –0.194 | .066 | — | — |
| Glomerular filtration rate 1 year post-HT, mL/min | –0.293 | .004 | –0.269 | .008 |
| Right atrial pressure 1 year post-HT, mmHg | +0.272 | .008 | +0.248 | .014 |
| TAPSE 1 year post-HT, mm | –0.183 | .077 | — | — |
| History of primary graft failure | +0.178 | .077 | — | — |
| Use of mycophenolate mofetil | –0.211 | .036 | –0.239 | .017 |
Gal-3, galectin-3; HT, heart transplant; TAPSE, tricuspid annulus plane systolic excursion.
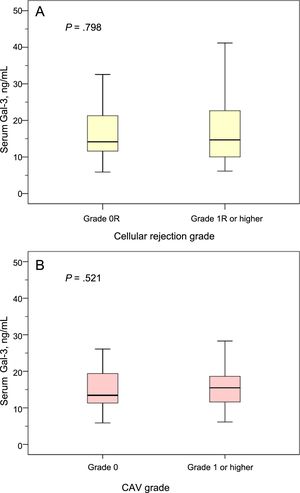
At the 1-year clinical visit, 117 patients (96%) underwent endomyocardial biopsy for assessment of graft rejection, and 31 of these patients (26%) showed a cellular rejection grade ≥ 1R. At this time point, 95 patients (78%) underwent coronary angiography for CAV assessment, and 17 of these patients (18%) showed a CAV grade of ≥ 1. The 1-year post-HT Gal-3 serum levels did not significantly differ between patients with or without a cellular rejection grade ≥ 1R (P=.521), or between patients with or without a CAV grade of ≥ 1 (P=.798; Figure 2).
Gal-3 serum levels according to cellular rejection grade and CAV grade. A: Gal-3 serum levels in patients with and without cellular rejection grade 1R or higher at the 1-year posttransplant clinical visit. B: Gal-3 serum levels in patients with and without CAV of grade ≥ 1 at the 1-year posttransplant clinical visit. CAV, coronary allograft vasculopathy; Gal-3, galectin-3.
A total of 99 patients had banked serum samples extracted at 1 year after HT, and these participants were followed up for a median of 6.7 years (IQR, 4.3–9.4) beyond this time point. The clinical characteristics of the study patients according to their Gal-3 serum levels 1 year after HT are detailed in .
During this period, 22 patients (22%) were hospitalized due to graft failure and 17 patients (17%) died. The causes of admission due to graft failure were CAV (n=6), cellular rejection (n=5), CAV and cellular rejection (n=4), antibody-mediated rejection (n=4), constrictive pericarditis (n=1), cardiac amyloidosis (n=1), and unknown (n=1). The causes of death were graft failure (n=6), sudden cardiac death (n=5), cancer (n=4), liver cirrhosis (n=1), and dementia (n=1).
Univariable Cox regression analysis revealed that 1-year posttransplant Gal-3 serum levels were significantly associated with a higher risk of the major composite outcome of all-cause death or graft failure over subsequent follow-up (HR per ng Gal-3/mL, 1.04; 95%CI, 1.01 – 1.06; P=.004). Gal-3 serum levels at 1 year post-HT were also associated with a higher risk of each individual component of the composite outcome, all-cause death (HR per ng Gal-3/mL, 1.05; 95%CI, 1.02 – 1.08; P=.002) and graft failure (HR per ng Gal-3/mL, 1.03, 95%CI, 1.01 – 1.07; P=.020).
After multivariable adjustment (), 1-year posttransplant Gal-3 serum levels retained an independent statistically significant association with a higher risk of the composite outcome (adjusted HR per ng Gal-3/mL, 1.04; 95%CI, 1.01 – 1.06; P=.008), and with higher risk of the 2 individual components, all-cause death (adjusted HR per ng Gal-3/mL, 1.15; 95%CI, 1.07 – 1.25; P<.001) and graft failure (adjusted HR per ng Gal-3/mL, 1.06; 95%CI, 1.01 – 1.09; P=.010).
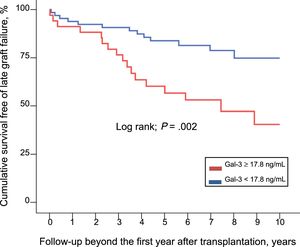
Figure 3 shows the cumulative long-term survival free of graft failure among patients with 1-year posttransplant Gal-3 serum levels above the reference range (≥ 17.8 ng/mL), compared with those with normal levels (< 17.8 ng/mL). By means of Cox multivariable regression (), the estimated adjusted HR for the major composite outcome of all-cause death or graft failure was 2.74 (95%CI, 1.07 – 7.02; P=.035).
Predictive Value of Posttransplant Gal-3 Serum LevelsFigure 4 shows the ROC curve constructed to investigate how accurately 1-year posttransplant Gal-3 serum levels could predict the major composite outcome of all-cause death or graft failure, and of its 2 individual components, over subsequent long-term follow-up. The area under the ROC curve was 0.72 (95%CI, 0.60-0.82; P<.001) for the major composite outcome, 0.76 (95%CI, 0.64-0.88; P<.001) for all-cause mortality, and 0.67 (95%CI, 0.54-0.80; P=.014) for graft failure. The preselected Gal-3 serum level cutoff value (≥ 17.8 ng/mL) detected the occurrence of the major combined outcome, all-cause mortality, and late graft failure, with sensitivities of 57%, 65%, and 55%; and specificities of 75%, 72%, and 71%, respectively.
DISCUSSIONThe major finding of this study is that serum levels of Gal-3, a known marker of inflammation, fibrosis, and adverse outcomes in patients with HF, showed a steady decreasing trend during the first year after HT. This decline likely reflected a progressive reversal of the preoperative state of HF after transplant. Moreover, this is the first study to support the prognostic value of serum Gal-3 in HT recipients. Within our cohort, patients who exhibited persistently elevated Gal-3 levels at the end of the first year after transplant showed significantly worse clinical outcomes over subsequent long-term follow-up.
Our results showing a declining trend of Gal-3 serum levels after HT differed from the findings of Grupper et al.9 In their study, they determined Gal-3 serum levels before and after HT among 62 patients with advanced HF at the Mayo Clinic. Although the mean pretransplant Gal-3 serum levels were consistent with those described in our population (20.3 ng/mL in the American study vs 19.1 ng/mL in our study), they did not observe a significant postoperative decrease. They concluded that serum Gal-3 levels strongly depend on the development of posttransplant renal function, and questioned the usefulness of this biomarker in HT recipients. Another study examined 85 stable HT recipients and reported mean posttransplant Gal-3 serum levels of 17.8 ng/mL, which were similar to the mean value determined in a parallel cohort of 55 patients with stable HF but were significantly lower than for a parallel cohort of 63 patients with severe HF.8
The discrepancy between our results and the findings of Grupper et al.9 are likely due to differences in the populations examined, as well as the different time-points for Gal-3 serum level measurement following HT. In the American study,9 there was a high prevalence of restrictive cardiomyopathy among HT candidates, probably because it was conducted in a large referral center for this specific cardiac disorder. In addition, there was wide interindividual variability in the interval from HT to serum Gal-3 measurement, with an IQR of 54-767 days. In contrast, we performed measurements at fixed time-points for all participants throughout the first year after transplant. This was an important difference, since a newly implanted heart graft has a progressive rather than an immediate effect with regards to normalizing hemodynamics and reversing the pathophysiological disturbances and end-organ dysfunction inherent to the preoperative, sometimes long-standing, systemic HF state. Moreover, in the early post-HT stages, recipients may show substantial systemic inflammatory activity caused by several triggers, such as surgical aggression, organ cold ischemia, blood transfusions, infection, and host-to-graft immune responses. Consistent with these arguments, our present findings did not indicate that Gal-3 serum levels immediately declined following HT. Compared with preoperative values, a significant decrease in Gal-3 serum level was first detected at 6 months post-HT.
Our results identified several clinical variables that were associated with posttransplant Gal-3 serum levels. Similar to previous reports,3,9,13 serum Gal-3 was inversely correlated with glomerular filtration rate, likely due to decreased biomarker clearance in patients with renal dysfunction. Post-HT Gal-3 serum levels were positively correlated with high right atrial pressure, a sensitive marker of heart graft dysfunction and a major determinant of cardio-renal syndrome.14 The use of mycophenolate mofetil was associated with lower posttransplant Gal-3 serum levels, which was consistent with the beneficial effects of this therapy on immune tolerance, graft function, and overall survival of HT recipients.15 We also found that lower donor age was associated with increased posttransplant serum Gal-3, which we could not explain. By contrast, previous studies have consistently shown that heart grafts from younger donors are less prone to failure than those from older donors.
The present study supports the strong prognostic value of serum Gal-3 in HT recipients. Patients with 1-year posttransplant Gal-3 serum levels above the reference limit of 17.8 ng/mL exhibited > 3-times the risk of all-cause death or hospitalization due to graft failure (ie, symptomatic HF caused by graft dysfunction) over subsequent long-term follow-up. This independent association between posttransplant serum Gal-3 and long-term clinical outcomes remained significant even after extensive multivariable adjustment for several clinical characteristics, including renal function.
Our findings are consistent with the previously reported prognostic value of serum Gal-3 in several subsets of patients with cardiovascular disease, especially among patients with HF.4,5 However, there remains a need for further examination of the possible reasons why persistently elevated Gal-3 serum levels are associated with increased risk of death and graft failure in HT recipients. Gal-3 serum level has been proposed as a cardiac biomarker based on its direct correlation with the grade of inflammation and, more importantly, fibrosis of cardiac tissue.1–3,16 Thus, it could be hypothesized that persistently elevated serum Gal-3 after HT may be a sensitive indicator of subtle subclinical structural changes in the heart graft reflected in the host immunological responses. In our study, the 1-year posttransplant Gal-3 levels were not significantly associated with the presence of cellular rejection or CAV at the same time point. However, this might be a reflection of the low sensitivity of the diagnostic tests used to detect graft rejection and CAV, endomyocardial biopsies and coronary angiography. Indeed, Coromilas et al.8 previously found that higher posttransplant Gal-3 serum levels were significantly associated with CAV occurrence.
Prior reports have questioned the clinical usefulness of serum Gal-3 as a cardiac biomarker due to its relative lack of specificity and its dependence on other biological variables, mainly renal function.8,11 In this respect, Gal-3 serum levels might reflect the grade of inflammation and fibrosis at biological levels other than the cardiovascular system, such as in the kidneys, liver, or pancreas.1 Notably, significantly increased serum Gal-3 has also been described in patients with some types of cancer.17 In patients with chronic HF, Gal-3 serum levels correlate with the severity of end-organ dysfunction. Therefore, persistent serum Gal-3 elevation after HT may at least partly result from the inability of the newly implanted heart graft to reverse the systemic pathophysiological abnormalities inherent in the preexisting HF syndrome. Notably, in our study, serum Gal-3 predicted all-cause mortality more accurately than graft failure, arguing against its specificity as a cardiac biomarker.
This study has several limitations. First, it was an observational study for which clinical data were collected retrospectively; therefore, the results are subject to potential selection, information, and confusion biases. Second, although the total study population included 122 HT recipients, banked serum samples for Gal-3 measurement were only available from 99 to 105 patients, depending on the time point. Third, CAV and acute rejection were detected based on endomyocardial biopsies and coronary angiographies, respectively, which were scheduled according to the standard clinical follow-up protocol of our center. We did not routinely use other more sensitive methods, such as intravascular ultrasound, which might have resulted in a significant number of false-negative diagnoses in our study. Fourth, there is presently a lack of information regarding the posttransplant dynamics of other clinically relevant biomarkers, such as NT-pro-BNP, and a comparison was not part of this study. Finally, we recognize that our statistical approach to the predictive accuracy of serum Gal-3 levels in HT recipients has limited value, and it should be confirmed through external validation in a larger, prospective cohort.
CONCLUSIONSThe results of this study demonstrate than Gal-3 serum levels steadily decreased over the first year after HT, with a significant reduction beyond 6 months posttransplant. Furthermore, HT recipients with persistently elevated Gal-3 serum levels at 1-year posttransplant exhibited lower graft failure-free survival during subsequent long-term follow-up. Remarkably, Gal-3 serum levels correlated better with the risk of all-cause mortality than with the risk of graft-related events, CAV, rejection, or hospitalization due to HF; this finding suggest that it might be considered as a systemic rather than a cardiac specific biomarker. Future prospective multi-institutional studies are warranted to clarify the value of serum Gal-3 as a biomarker in the routine clinical management of HT recipients.
FUNDINGThis study was funded by the Project N° PI12/02670, integrated in the National Plan for Scientific Research, Development and Technological Innovation 2008-2011 and funded by the ISCIII (Instituto de Salud Carlos III) General Subdirection of Assessment and Promotion for Research-ERDF (European Regional Development Fund) “A way of making Europe”.
CONFLICTS OF INTERESTNone declared.
- –
Serum Gal-3 is a marker of cardiac and systemic fibrosis that has been associated with poor outcomes in patients with HF; however, its long-term profile and prognostic value in HT recipients are unknown. Until now, only 1 published study has described the trend of serum Gal-3 after HT, showing no significant decrease in this parameter after the intervention.
- –
In our cohort, serum Gal-3 levels followed a descending trend during the first year after HT, probably as a result of the progressive resolution of the systemic HF state after the intervention. In addition, our study suggests that persistently elevated serum Gal-3 levels 1 year after HT were associated with lower graft failure-free survival in the long-term.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2018.10.005.