The management of atrial fibrillation is complex and requires improvement at strategic points, such as in the control of patients treated with vitamin K antagonists. The aim of this study was to evaluate the impact on health outcomes of a nonvalvular atrial fibrillation decision support tool based on visualization of the time in therapeutic range in primary care.
MethodsThe present randomized clinical trial was conducted in 2018 with a 1-year follow-up in 325 primary care centers in Catalonia. In the intervention centers, the decision support tool was installed to control the time in therapeutic range of patients treated with vitamin K antagonists. The tool was not visualized in the control group.
ResultsIn total, 44 556 patients were studied. The intervention protected against admission for stroke (adjusted odds ratio [OR], 0.70; 95% confidence interval [95%CI], 0.55-0.88). The number needed to treat was 3502 (95%CI, 3305-3725) while the number of admissions for stroke avoided was 12.63 (95%CI, 11.88-13.38). The intervention also protected against mortality (adjusted OR, 0.78; 95%CI, 0.67-0.90), with a number needed to treat of 13 687 (95%CI, 10 789-18 714) and number of deaths avoided of 3.23 (95%CI, 2.36-4.10).
ConclusionsThe decision support tool was associated with slight reductions in the numbers of admissions for ischemic stroke and mortality. Although the follow-up time was short and the effect of the intervention was small, the results are valuable and could improve implementation of the tool.
This clinical trial was registered with ClinicalTrials.gov (NCT03367325).
Keywords
The prevalence of atrial fibrillation in adults in Spain is currently estimated to be between 2% and 4% rising to 4.4% in those older than 40 years of age.1,2 The morbidity most closely associated with atrial fibrillation is ischemic stroke,3 and there were about 12.2 million incident cases of stroke and 6.5 million deaths from stroke worldwide in 2019.4 Stroke associated with atrial fibrillation accounts for approximately 25% of all strokes and causes the highest disability.3
Oral anticoagulants are the drugs of choice to prevent stroke in atrial fibrillation.5 In the case of nonvalvular atrial fibrillation, 2 types of oral anticoagulants can be administered to prevent a thromboembolic event: vitamin K antagonists and direct-acting oral anticoagulants.6
Current European guidelines for the management of nonvalvular atrial fibrillation recommend the use of direct-acting anticoagulants and, if there is a contraindication, vitamin K antagonists.6 Vitamin K antagonists were the most widely used anticoagulants in Spain in 2018, representing 57.7%.7,8 Currently, direct-acting anticoagulants are used in 58.1% of patients in Spain, in 59.0% of those in Catalonia, and in about 80.0% of those in Europe.9
Some strategic points in the treatment of nonvalvular atrial fibrillation with anticoagulants require improvement. One of the problems is that patients who receive vitamin K antagonists cannot adequately control the time in therapeutic range (TTR).10 This can be improved not only by knowing the international normalized ratio (INR), which determines the degree of anticoagulation control at a given moment, but also by enabling health care professionals to access Rosendaal's11 automatically calculated TTR as an intrinsic part of their patients’ electronic medical records. This variable measures the length of time in which patients had good control in the last 6 months and is needed to make decisions about anticoagulant treatment switching. It can also help to optimize control with vitamin K antagonists when patients do not adhere to their treatment.
Support systems for clinical decision-making are increasingly common in electronic medical records. Although these systems appear to improve the care processes for different pathologies, there is little evidence that they improve clinical or economic outcomes.12 In clinical practice, these tools do not reduce mortality when integrated into electronic medical records, although they may moderately reduce morbidity rates.13 In recent years, several studies have developed tools for decision-making for controlling atrial fibrillation. Most of these solutions focus on anticoagulation initiation after an atrial fibrillation diagnosis.14,15 Some of these tools improve adherence to clinical practice guidelines13 and lead to a slightly better reduction in bleeding.14
Given that patients treated with vitamin K antagonists have poor anticoagulation control, it would be useful to know the TTR in primary care, where most patients are managed. In this context, a decision-making tool to visualize the TTR in primary care could improve health outcomes. Thus, the objective of this study was to evaluate the impact on health outcomes of the nonvalvular atrial fibrillation clinical decision support tool (CDS-NVAF) in primary care electronic medical records, based on visualization of the TTR.
METHODSStudy design and populationThe study design has previously been published.16 The general design of the study and the deviations from the study protocol are described below. This clinical trial was registered with ClinicalTrials.gov (NCT03367325).
A parallel-group, randomized clinical trial was conducted, with randomization by primary care center sector.16 A sector is a group of primary care centers sharing the same server. The Catalan Health Institute has 15 sectors covering 325 primary care centers. The sectors were randomized by the data extractors, using the simple 1:1 randomization method, and with numbers of 0 and 1 being randomly generated. Patients with a value<0.5 were assigned to the control group while those with a value ≥ 0.5 were assigned to the intervention group. The study was blinded to the trial patients, project investigators, and data managers, but not to the health care professionals (figure 1).
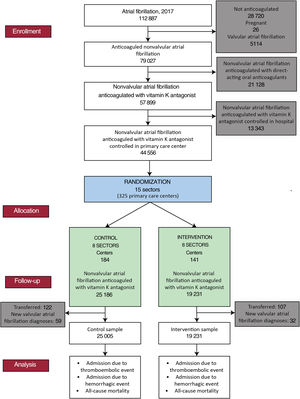
The study variables were collected from the population database of the Information System for the Development of Primary Care Research.16 In January 2017, 112 887 people with an active diagnosis of atrial fibrillation were detected (figure 2). The study included patients with at least a 1-year history of nonvalvular atrial fibrillation by January 2018 who had been receiving anticoagulant treatment with vitamin K antagonists (acenocoumarol or warfarin) for at least 6 months at the beginning of the study and who had at least 6 monthly INR measurements in the last 6 months of 2017. They were considered to be receiving anticoagulant treatment with vitamin K antagonists if they had an active prescription at the beginning of the study and 2 months before. They were considered to have changed their anticoagulant if they had received more than 2 months of treatment with direct-acting anticoagulants (dabigatran, rivaroxaban, apixaban, or edoxaban) in 2018.
Patients with criteria of valvular atrial fibrillation, presenting as mitral stenosis or with a prosthetic cardiac valve (text 1 of the supplementary data) were excluded. Also excluded were nonanticoagulated patients, pregnant women, patients who were receiving treatment with direct-acting anticoagulants at the beginning of the study, and those who were followed up at the referral hospital. Losses to follow-up caused by transfers of patients to another health care system and new diagnoses of valvular atrial fibrillation in the included patients were discounted from the analysis (figure 2). An intention-to-treat analysis was conducted 1 year after the start of the intervention and according to patients’ characteristics.
All primary care centers of the Catalan Health Institute of Catalonia were eligible to participate in the study. The moment of implementation of the CDS-NVAF tool to initiate the clinical trial was determined by the Directorate of Primary Care of the Catalan Health Institute, who informed the territorial directors of the start of the study. Deviations from the protocol and a description of the final version of the CDS-NVAF are included in texts 2 and 3 of the supplementary data.
Sample sizeThe sample comprised 44 556 individuals with nonvalvular atrial fibrillation who were anticoagulated with vitamin K antagonists and who were treated within the primary care system. Of these, 25 186 (from 184 health centers) and 19 370 (from 141 health centers) were in the control and intervention groups, respectively. Assuming 90% power and a 5% alpha error, corrected for the clustered design, our study was able to detect a 0.5% difference for a stroke rate of 1.32×100 admissions per stroke per patient per year.17
Statistical analysisStatistically significant differences between the control and intervention groups were identified with Z tests for categorical variables and with nonparametric Mann-Whitney U tests for continuous variables. The incidences of admissions (with 95% confidence intervals [95%CIs]) due to thromboembolic and hemorrhagic events and mortality were calculated. Significant differences between the proportions in the control and intervention groups were detected with the Z test. The effect of the intervention (and the 95%CI) was estimated using Cohen's h for proportions (small effect, h<0.2; large effect, h>0.8).
The associations between the odds of death, admission for stroke, and the variables related to these events were quantified by using multivariate logistic regression to estimate odds ratios (ORs). The average risk difference, the number needed to treat, and the number of events avoided were calculated.18
The threshold for statistical significance was set at 5%. Statistical analyses were conducted with R software version 4.0.2 (R Foundation for Statistical Computing, Austria).
RESULTSThere was no difference between the control and intervention groups in loss to follow-up due to patient transfer (122 of 25 186 patients vs 107 of 19 370 patients; P=.320) and in new diagnoses of valvular atrial fibrillation (59 of 25 186 patients vs 32 of 19 370 patients; P=.109) (figure 2). Thus, at the end of follow-up, 25 005 and 19 321 patients from the control and intervention groups, respectively, could be analyzed.
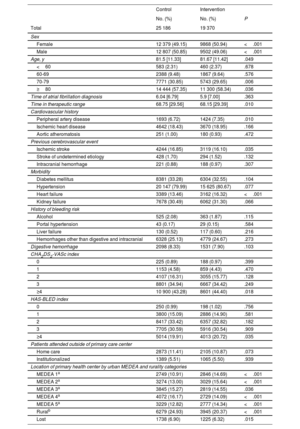
At baseline, the intervention group had higher proportions of women and of patients with a history of peripheral arterial disease and heart failure, higher scores (≥4) on the CHA2DS2-VASc and HAS-BLED scales, and a higher median age vs the control group. In contrast, the control group had a higher proportion of patients with a history of ischemic stroke and a longer median TTR. Patients in the intervention group were more likely to attend a primary care center in areas in deprivation quintiles 1, 2, and 5, while those in the control group were more likely to attend centers in quintiles 3 and 4. Control patients were more likely to live in rural areas than intervention patients (table 1). With the exception of median age, the differences in all variables persisted when patients lost to follow-up were excluded (table 1 of the supplementary data).
Characteristics of the baseline population
| Control | Intervention | ||
|---|---|---|---|
| No. (%) | No. (%) | P | |
| Total | 25 186 | 19 370 | |
| Sex | |||
| Female | 12 379 (49.15) | 9868 (50.94) | <.001 |
| Male | 12 807 (50.85) | 9502 (49.06) | <.001 |
| Age, y | 81.5 [11.33] | 81.67 [11.42] | .049 |
| <60 | 583 (2.31) | 460 (2.37) | .678 |
| 60-69 | 2388 (9.48) | 1867 (9.64) | .576 |
| 70-79 | 7771 (30.85) | 5743 (29.65) | .006 |
| ≥80 | 14 444 (57.35) | 11 300 (58.34) | .036 |
| Time of atrial fibrillation diagnosis | 6.04 [6.79] | 5.9 [7.00] | .363 |
| Time in therapeutic range | 68.75 [29.56] | 68.15 [29.39] | .010 |
| Cardiovascular history | |||
| Peripheral artery disease | 1693 (6.72) | 1424 (7.35) | .010 |
| Ischemic heart disease | 4642 (18.43) | 3670 (18.95) | .166 |
| Aortic atheromatosis | 251 (1.00) | 180 (0.93) | .472 |
| Previous cerebrovascular event | |||
| Ischemic stroke | 4244 (16.85) | 3119 (16.10) | .035 |
| Stroke of undetermined etiology | 428 (1.70) | 294 (1.52) | .132 |
| Intracranial hemorrhage | 221 (0.88) | 188 (0.97) | .307 |
| Morbidity | |||
| Diabetes mellitus | 8381 (33.28) | 6304 (32.55) | .104 |
| Hypertension | 20 147 (79.99) | 15 625 (80.67) | .077 |
| Heart failure | 3389 (13.46) | 3162 (16.32) | <.001 |
| Kidney failure | 7678 (30.49) | 6062 (31.30) | .066 |
| History of bleeding risk | |||
| Alcohol | 525 (2.08) | 363 (1.87) | .115 |
| Portal hypertension | 43 (0.17) | 29 (0.15) | .584 |
| Liver failure | 130 (0.52) | 117 (0.60) | .216 |
| Hemorrhages other than digestive and intracranial | 6328 (25.13) | 4779 (24.67) | .273 |
| Digestive hemorrhage | 2098 (8.33) | 1531 (7.90) | .103 |
| CHA2DS2-VASc index | |||
| 0 | 225 (0.89) | 188 (0.97) | .399 |
| 1 | 1153 (4.58) | 859 (4.43) | .470 |
| 2 | 4107 (16.31) | 3055 (15.77) | .128 |
| 3 | 8801 (34.94) | 6667 (34.42) | .249 |
| ≥4 | 10 900 (43.28) | 8601 (44.40) | .018 |
| HAS-BLED index | |||
| 0 | 250 (0.99) | 198 (1.02) | .756 |
| 1 | 3800 (15.09) | 2886 (14.90) | .581 |
| 2 | 8417 (33.42) | 6357 (32.82) | .182 |
| 3 | 7705 (30.59) | 5916 (30.54) | .909 |
| ≥4 | 5014 (19.91) | 4013 (20.72) | .035 |
| Patients attended outside of primary care center | |||
| Home care | 2873 (11.41) | 2105 (10.87) | .073 |
| Institutionalized | 1389 (5.51) | 1065 (5.50) | .939 |
| Location of primary health center by urban MEDEA and rurality categories | |||
| MEDEA 1a | 2749 (10.91) | 2846 (14.69) | <.001 |
| MEDEA 2a | 3274 (13.00) | 3029 (15.64) | <.001 |
| MEDEA 3a | 3845 (15.27) | 2819 (14.55) | .036 |
| MEDEA 4a | 4072 (16.17) | 2729 (14.09) | <.001 |
| MEDEA 5a | 3229 (12.82) | 2777 (14.34) | <.001 |
| Ruralb | 6279 (24.93) | 3945 (20.37) | <.001 |
| Lost | 1738 (6.90) | 1225 (6.32) | .015 |
CHA2DS2-VASc, thromboembolic risk score; HAS-BLED, hemorrhagic risk scale; IQR, interquartile range; P, significance of Z test of proportions.
Values are expressed as No. (%) or median [IQR].
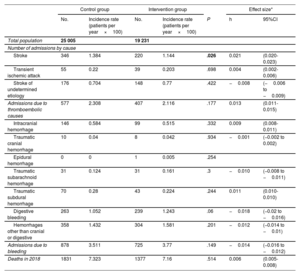
The incidence of admissions for stroke was lower in the intervention group than in the control group (1.14 vs 1.38 admissions per stroke per patient per year×100; P=.026). No differences were detected in the incidence of any other thromboembolic or hemorrhagic events between the 2 groups. The Cohen's h effect size of the intervention for admission for stroke was 0.021 (95%CI, 0.020-0.023) (table 2).
Primary results of admissions due to thromboembolic and hemorrhagic events and death
| Control group | Intervention group | Effect size* | |||||
|---|---|---|---|---|---|---|---|
| No. | Incidence rate (patients per year×100) | No. | Incidence rate (patients per year×100) | P | h | 95%CI | |
| Total population | 25 005 | 19 231 | |||||
| Number of admissions by cause | |||||||
| Stroke | 346 | 1.384 | 220 | 1.144 | .026 | 0.021 | (0.020-0.023) |
| Transient ischemic attack | 55 | 0.22 | 39 | 0.203 | .698 | 0.004 | (0.002-0.006) |
| Stroke of undetermined etiology | 176 | 0.704 | 148 | 0.77 | .422 | −0.008 | (−0.006 to −0.009) |
| Admissions due to thromboembolic causes | 577 | 2.308 | 407 | 2.116 | .177 | 0.013 | (0.011-0.015) |
| Intracranial hemorrhage | 146 | 0.584 | 99 | 0.515 | .332 | 0.009 | (0.008-0.011) |
| Traumatic cranial hemorrhage | 10 | 0.04 | 8 | 0.042 | .934 | −0.001 | (−0.002 to 0.002) |
| Epidural hemorrhage | 0 | 0 | 1 | 0.005 | .254 | ||
| Traumatic subarachnoid hemorrhage | 31 | 0.124 | 31 | 0.161 | .3 | −0.010 | (−0.008 to −0.011) |
| Traumatic subdural hemorrhage | 70 | 0.28 | 43 | 0.224 | .244 | 0.011 | (0.010-0.010) |
| Digestive bleeding | 263 | 1.052 | 239 | 1.243 | .06 | −0.018 | (−0.02 to −0.016) |
| Hemorrhages other than cranial or digestive | 358 | 1.432 | 304 | 1.581 | .201 | −0.012 | (−0.014 to −0.01) |
| Admissions due to bleeding | 878 | 3.511 | 725 | 3.77 | .149 | −0.014 | (−0.016 to −0.012) |
| Deaths in 2018 | 1831 | 7.323 | 1377 | 7.16 | .514 | 0.006 | (0.005-0.008) |
P, significance of Z test of proportions.
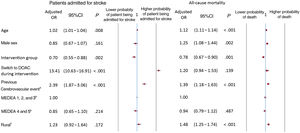
Stroke admissions were associated with an increasing age (adjusted OR, 1.02; 95%CI, 1.01-1.04), a switch from a vitamin K antagonist to a direct-acting anticoagulant (adjusted OR, 13.41; 95%CI, 10.63-16.91), and a history of stroke (adjusted OR, 2.39; 95%CI, 1.87-3.06), including a stroke of undetermined etiology and an intracranial hemorrhage before the start of the intervention. Members of the intervention group were protected against admission for stroke (adjusted OR, 0.70; 95%CI, 0.55-0.88) (figure 3), and the CDS-NVAF tool would need to be used on 3502 (95%CI, 3305-3725) patients to prevent 1 patient from being admitted for stroke. Overall, 12.63 (95%CI, 11.88-13.38) events were avoided (table 3).
Factors associated with admissions for stroke and all-cause mortality.
95%CI, 95% CI, confidence interval; DOAC, direct-acting oral anticoagulant.
a Previous cerebrovascular event includes stroke, a stroke of undetermined etiology, and intracranial hemorrhage.
b MEDEA is an index of material and social deprivation (1, low deprivation; 5, high deprivation) for the location of urban primary care centers attended by patients.
c Rural refers to primary health care centers serving rural populations.
Impact measures of the nonvalvular atrial fibrillation decision support tool
| Primary outcome | OR (95%CI)* | ARD (95%CI) | NNT (95%CI) | Number of events avoided (95%CI) |
|---|---|---|---|---|
| Patients admitted for stroke | 0.70 (0.55-0.88) | 2.86×10−4 (2.66×10−4-3.05×10−4) | 3502 (3305-3725) | 12.63 (11.88-13.38) |
| Mortality | 0.78 (0.67-0.90) | 7.30×10−5 (5.34×10−5-9.26×10−5) | 13 687 (10 789-18 714) | 3.23 (2.36-4.10) |
ARD, average risk difference; CI, confidence interval; NNT, number needed to treat; OR, odds ratio
Higher odds of death were associated with an older age (adjusted OR, 1.12; 95%CI, 1.11-1.14), male sex (adjusted OR, 1.25; 95%CI, 1.08-1.44), a history of stroke (adjusted OR, 1.39; 95%CI, 1.18-1.63), and a rural health center (adjusted OR, 1.48; 95%CI, 1.25-1.74). Being in the intervention group protected against death (adjusted OR, 0.78; 95%CI, 0.67-0.90) (figure 3) and the CDS-NVAF tool would need to be used on 13 687 (95%CI, 10 789-18 714) patients to prevent 1 death. In total, 3.23 (95%CI, 2.36-4.10) events were prevented (table 3).
The TTR (≥65%) and TTR (>70%) did not improve control at 1 year of follow-up in the intervention group compared with the control group. A switch to direct-acting oral anticoagulants was more likely in the intervention group (table 2 of the supplementary data).
DISCUSSIONThe present study examined a decision support tool integrated into electronic medical records. The CDS-NVAF tool allows primary care professionals to assess the degree of anticoagulation control in patients with nonvalvular atrial fibrillation who are anticoagulated with vitamin K antagonists. This tool led to a slight decrease in the number of admissions for stroke and may also have been associated with a drop in mortality. The impact of the intervention was favorable with respect to the avoidance of admissions for stroke and the mortality rate, and we can thus conclude that implementation of the tool may yield improvements.
The patients who can benefit from the CDS-NVAF tool are those receiving anticoagulation therapy with vitamin K antagonists. These drugs have a narrow therapeutic window and the response to treatment varies greatly among individuals. This makes it necessary to monitor anticoagulation and dose adjustments based on the INR, which corresponds to a standardized calculation of prothrombin time. There is a consensus that patients with nonvalvular atrial fibrillation treated with vitamin K antagonists should maintain an INR between 2 and 3 for a long as possible to achieve a high TTR.6 These recommendations are based on the ability of these anticoagulants to prevent thromboembolic events over these ranges and on the increase in adverse hemorrhagic effects from an INR ratio of 4.19 The current European guidelines for atrial fibrillation consider a TTR >70% at 6 months to be good control.6 However, because the Spanish guidelines consider a TTR ≥ 65% to represent good control,20 we used this value in our study. Poorly controlled TTR is a parameter used to decide a switch from a vitamin K antagonist to a direct-acting anticoagulant, provided that good adherence to treatment has been verified.
Regarding health outcomes, admissions for stroke were less frequent in the intervention group than in the control group, although the effect was small, with incidences of 1.14 and 1.38 admissions for stroke per 100 patients per year, respectively. Studies with a longer follow-up time showed similar incidences of stroke in a warfarin-treated group (1.32-1.66 strokes per 100 patients per year) to that of our control group. These studies showed a reduction in the risk of stroke with direct-acting anticoagulants of 0.97 to 1.33 strokes per 100 patients per year, a rate closer to that noted in our intervention group.17,21
The CDS-NVAF had little effect on reducing stroke admissions. This could be because the tool was introduced in a randomized clinical trial format in accordance with standard clinical practice. The tool was installed and announced in the electronic medical records as standard for similar innovations but the information provided might not have been sufficient to ensure the proper understanding and use of the tool. However, the observed effectiveness is based on real practice and not on the efficacy of randomized clinical trials conducted under ideal conditions. In addition, there was no improvement in the TTR in the intervention group, meaning that some of the beneficial effect of the tool could be attributed to the switch to direct-acting oral anticoagulants.
A study of mortality in anticoagulated individuals with a median age of between 70 and 73 years identified a mean mortality of 4.63 deaths per 100 patients per year.22 In our study of a sample of patients with a median age of 81 years, mortality rates were higher in the intervention group than in the control group (7.16 and 7.32 deaths per 100 patients per year, respectively). A recent study of mortality in a Spanish population of patients treated with vitamin K antagonists found all-cause mortality to be 6.14% for good anticoagulation control and 11.62% for poor control.23
The CDS-NVAF tool was associated with a reduction in admissions for stroke. Members of the CDS-NVAF intervention group were somewhat protected against admissions for stroke, with an adjusted OR of 0.70 (95%CI, 0.55-0.88). Conversely, factors associated with an increased likelihood of admission for stroke were age, a switch to direct-acting anticoagulant treatment during the study period, and a history of cerebrovascular disease. Age and a history of cerebrovascular disease are well-established factors that increase the risk of stroke in individuals with atrial fibrillation. Age is the most influential factor for those aged 75 years or more. Evidence suggests that a history of stroke may be the most important factor determining the occurrence of a new stroke.24 According to our results, the presence of a history of cerebrovascular disease increased the adjusted odds of admission for stroke by 2.39 times (95%CI, 1.87-3.06). This outcome was taken into account to adjust for the initial differences between the 2 groups of ischemic strokes. The recurrence rate was lower for ischemic small-vessel strokes and has declined in the last decade. In contrast, cardioembolic strokes, most of which are caused by atrial fibrillation, had a 54% risk of recurrence and their incidence has stabilized over the last decade. These facts highlight the value of implementing effective preventive strategies in atrial fibrillation patients to prevent thromboembolic stroke.25
On the other hand, in our study, the factor showing the strongest association with admission for stroke was an anticoagulant switch during the intervention period (adjusted OR, 13.43; 95%CI, 10.63-16.91). The type of analysis conducted here does not allow us to determine the cause of a stroke related to changes in anticoagulant treatment. Therefore, the stroke could be caused by a previous change to a type of anticoagulant associated with a greater risk of stroke during the initial months or because the admission for stroke happened before the anticoagulant was changed. Patients with nonvalvular atrial fibrillation who experience acute ischemic stroke are at risk of both hemorrhagic transformation and recurrent ischemic stroke in the poststroke period. The optimal time to anticoagulate patients with ischemic stroke is not known.26
The intervention was found to protect against admission for stroke. To our knowledge, this is the first real-life clinical trial of a decision support tool integrated within electronic medical records in order to support the management of atrial fibrillation that has reduced admissions for stroke.13,14 It should also be noted that this tool enables the better detection of candidates suitable for a switch to direct-acting anticoagulants. This may partially account for the observed reduction in the rates of stroke admission and perhaps of mortality.
The visualization of the TTR by the primary health care professionals reduced the risk of mortality after adjustment for other variables (adjusted OR, 0.78; 95%CI, 0.67-0.90). Two systematic reviews and meta-analyses designed to study the effectiveness of clinical decision support tools did not produce evidence of a mortality reduction.12,27 Here, higher odds of mortality were also associated with older age, male sex, a history of cerebrovascular disease, and attending a rural primary health center. Previous studies have confirmed that all-cause mortality is higher in rural than urban areas and is related to health inequalities and their determinants.28,29 The main element underlying urban and rural inequalities is the lower rural life expectancy.29 An important factor in this urban-rural gap is cardiovascular disease, and stroke in particular, with a 30% higher mortality rate from stroke in rural than urban areas.30
The results are likely to have external validity at the level of the public health systems of other regions of Spain and probably for primary public health care systems throughout Europe. Current evidence indicates that visualization of the TTR calculation is an essential tool for controlling anticoagulation in hospital settings and in primary care.31,32 Future studies should determine whether the CDS-NVAF tool was visualized and understood by the health care professionals. Its extension should be reinforced by ensuring that the health care professionals are trained in its use.
LimitationsOne of the limitations of the study lies in the baseline differences between the intervention and control groups. Because the work comprised a large-scale population study, the differences between the proportions in the 2 groups were statistically significant, even though they were small and of little substantive relevance. Second, it was not possible to obtain sociodemographic data on the health care professionals because of data protection constraints; this prevented a multilevel analysis being undertaken to identify the characteristics of the health care professionals that are associated with better health outcomes. Third, although fewer patients were included than estimated in 2015, this did not affect the power of the study, given the size of the final sample. While the effect of the intervention was small and the follow-up time was short, the results are population-based and consistent. In addition, reducing vitamin K antagonist use could reduce the impact of the tool. Fourth, the results are not disaggregated by sex but will be presented with the proportions of men and women and the main result includes sex as a predictor. Finally, the design of the CDS-NVAF does not allow confirmation that the health care professional viewed the TTR and followed the recommendation, based on the TTR percentage, to confirm adherence or to change anticoagulant. Accordingly, it is essential to know the opinions of the professionals treating the intervention group regarding the barriers to understanding the tool.
CONCLUSIONSThe impact of the intervention was favorable in terms of avoiding admissions for stroke and reducing mortality. The CDS-NVAF was associated with slight reductions in the admission rate for ischemic stroke and in mortality. No differences were found in the rates of hemorrhage. The CDS-NVAF tool is the first such approach to reduce admissions for stroke and mortality. Although the follow-up time was short and the effect of the intervention was modest, the results are nevertheless noteworthy. The effectiveness of the tool should be studied over a longer term to improve its implementation.
FUNDINGThis work was supported by the Department of Health of the Generalitat de Catalunya, Strategic Plan for Research and Innovation in Health (PERIS) (grant numbers, SLT002/16/00146 and SLT008/18/00021) and Carlos III Institute (grant number, PI21/01435).
ETHICAL CONSIDERATIONSThe study was performed according to national and international norms (Declaration of Helsinki) concerning ethical aspects. This study protocol was approved by the Ethical Committee of Clinical Investigation of the Institut Universitari d’Investigació en Atenció Primària (IDIAP) Jordi Gol, on March 15, 2017 (code P17/091). In addition, the study was authorized by the Directorate of Primary Care of the Catalan Health Institute. The data included in the Information System for the Development of Primary Care Research database were anonymized and were identified by an internal code, making subject identification impossible even by the investigative team. This guaranteed the confidentiality of the data of the study participants included in the study according to the Organic Law on Data Protection and Guarantee of Digital Rights.33 Because the study is based on the analysis of an anonymized database, informed consent was not collected from the patients. The ethics committee ruled that informed consent was not necessary.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used for the development of this work.
AUTHORS’ CONTRIBUTIONSConception and design of the study: M. R. Dalmau Llorca, C. Aguilar Martín, and A. Queiroga Gonçalves. Acquisition of funding: M. R. Dalmau Llorca, C. Aguilar Martín, N. Carrasco-Querol, Z. Hernández Rojas, D. Rodríguez Cumplido, E. Castro Blanco, A. Queiroga Gonçalves, J. Fernández-Sáez, and J. Pérez-Villacastín. Methodology: M. R. Dalmau Llorca, C. Aguilar Martín, D. Rodríguez Cumplido, A. Queiroga Gonçalves, and J. Fernández-Sáez. Data analysis: M. R. Dalmau Llorca, C. Aguilar Martín, Z. Hernández Rojas, E. Castro Blanco, and J. Fernández-Sáez. Data interpretation: M. R. Dalmau Llorca, C. Aguilar Martín, D. Rodríguez Cumplido, E. Castro Blanco, J. Fernández-Sáez, and J. Pérez-Villacastín. Statistical analysis: M. R. Dalmau Llorca, C. Aguilar Martín, Z. Hernández Rojas, E. Castro Blanco, and J. Fernández-Sáez. Manuscript drafting: M. R. Dalmau Llorca, C. Aguilar Martín, N. Carrasco-Querol, Z. Hernández Rojas, D. Rodríguez Cumplido, E. Castro Blanco, A. Queiroga Gonçalves, J. Fernández-Sáez, and J. Pérez-Villacastín. Manuscript review and editing: M. R. Dalmau Llorca, C. Aguilar Martín, N. Carrasco-Querol, Z. Hernández Rojas, D. Rodríguez Cumplido, E. Castro Blanco, A. Queiroga Gonçalves, J. Fernández-Sáez, and J. Pérez-Villacastín. Final review: M. R. Dalmau Llorca, C. Aguilar Martín, N. Carrasco-Querol, Z. Hernández Rojas, D. Rodríguez Cumplido, E. Castro Blanco, A. Queiroga Gonçalves, J. Fernández-Sáez, and J. Pérez-Villacastín.
CONFLICTS OF INTERESTM. R. Dalmau Llorca, C. Aguilar Martín, Z. Hernández Rojas, Dolores Rodríguez Cumplido, A. Queiroga Gonçalves, and J. Fernández-Sáez declare having received partial study funding from Bayer Hispania SL. The sponsor was not involved in the design and execution of the study; the collection, management, analysis, and interpretation of data; or the preparation, review, and approval of the manuscript or the decision to submit it for publication. Bayer Hispania SL provided technical support to the Catalan Health Institute to develop the TTR calculation in the electronic medical records but did not influence the design of the study or the visualization of the tool.
The authors thank the following departments for their contributions: Primary Care Management of the Catalan Institute of Health, Information Systems of the Primary Care Services, Regional Management and Primary Care Management of the Terres de l’Ebre, Information Systems Unit of the Regional Management Terres de l’Ebre, Functional Competences Center of the eCAP of the Information Systems Area, and SIDIAP.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2023.11.009.