The Spanish Society of Cardiology endorses the clinical practice guidelines (CPG) published by the European Society of Cardiology (ESC). As part of this policy, ESC guidelines are translated into Spanish and published in the online version of Revista Española de Cardiología¸ with the aim of increasing their accessibility and facilitating their implementation.1 The translated articles are accompanied by an editorial authored by a panel of Spanish experts that highlights the most important content of each CPG document, detailing changes and innovations introduced since the previous edition and discussing the more contentious aspects and possible limitations. The editorial also seeks to evaluate and adapt the recommendations to the context of health care organization and clinical practice in Spain.
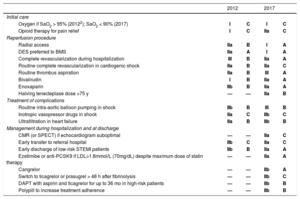
The latest ESC guidelines on ST-segment elevation myocardial infarction (STEMI)2 updates the previous CPG published in 20123 and presents 159 recommendations (58% in class I, 24% in class IIa, 8% in class IIb, and 10% in class III) based on 3 levels of evidence (23% in level A, 28% in level B, and 49% in level C). The recommendations are supported by 477 cited publications. The main changes with respect to the previous guidelines are summarized in the Table. It should be noted that strong efforts have been made to maintain coherence with previous guidelines.
Principal changes in the 2017 ESC CPG on STEMI2
| 2012 | 2017 | |||
|---|---|---|---|---|
| Initial care | ||||
| Oxygen if SaO2 > 95% (20122); SaO2 < 90% (2017) | I | C | I | C |
| Opioid therapy for pain relief | I | C | IIa | C |
| Reperfusion procedure | ||||
| Radial access | IIa | B | I | A |
| DES preferred to BMS | IIa | A | I | A |
| Complete revascularization during hospitalization | III | B | IIa | A |
| Routine complete revascularization in cardiogenic shock | IIa | B | IIa | C |
| Routine thrombus aspiration | IIa | B | III | A |
| Bivalirudin | I | B | IIa | A |
| Enoxaparin | IIb | B | IIa | A |
| Halving tenecteplase dose >75 y | — | — | IIa | B |
| Treatment of complications | ||||
| Routine intra-aortic balloon pumping in shock | IIb | B | III | B |
| Inotropic vasopressor drugs in shock | IIa | C | IIb | C |
| Ultrafiltration in heart failure | IIa | B | IIb | B |
| Management during hospitalization and at discharge | ||||
| CMR (or SPECT) if echocardiogram suboptimal | — | — | IIa | C |
| Early transfer to referral hospital | IIb | C | IIa | C |
| Early discharge of low-risk STEMI patients | IIb | B | IIa | A |
| Ezetimibe or anti-PCSK9 if LDL>1.8mmol/L (70mg/dL) despite maximum dose of statin therapy | — | — | IIa | A |
| Cangrelor | — | — | IIb | A |
| Switch to ticagrelor or prasugrel > 48 h after fibrinolysis | — | — | IIb | C |
| DAPT with aspirin and ticagrelor for up to 36 mo in high-risk patients | — | — | IIb | B |
| Polypill to increase treatment adherence | — | — | IIb | B |
BMS, bare-metal stent; CMR, cardiac magnetic resonance; DAPT, dual antiplatelet therapy; DES, metal drug-eluting stent; anti-PCSK9, inhibitory antibody to proprotein convertase subtilisin/kexin type 9; SPECT, single-photon emission computed tomography; STEMI, ST-segment elevation myocardial infarction.
Acute myocardial infarction (AMI) is defined by elevation of cardiac troponins in a clinical setting suggestive of myocardial ischemia; the presumptive diagnosis of STEMI is based on the coexistence of persistent symptoms suggesting ischemia and ST-segment elevation in 2 contiguous leads.4 The new guidelines affirm the declining incidence of STEMI and the progressive reduction in associated mortality, which has also been documented in Spain.5 These improvements are likely attributable to the more widespread use of reperfusion therapies and other recommended treatments. The CPG authors also highlight the high prevalence of STEMI in the elderly and in women and comment on the diagnostic difficulties encountered in these populations.
In emergency care, the guidelines advise against the use of the nitroglycerin response for diagnosis and discuss the implications of difficult electrocardiography (ECG) patterns. Patients with persistent ischemia and bundle branch block (whether or not identified as right or left) should receive standard STEMI therapy: immediate angiography and percutaneous coronary intervention (PCI) if indicated. Atypical ECG patterns are defined, including those for bundle branch block, ventricular pacing, hyperacute T-waves, isolated ST-depression in anterior leads, and diffuse ST-depression with ST-elevation in aVR.
Several important implications emerge from the expansion of the indications for emergency catheterization among patients with AMI symptoms but atypical ECG patterns. The assignation of right bundle branch block as an indication for immediate angiography is based on a study showing a high incidence (67%) of TIMI flow < 3 in the infarction-related artery in AMI patients with right bundle block without ST-segment elevation.6 However, this was a retrospective study that provided no information about patient symptoms or the presence of ST-segment depression suggestive of posterior AMI. Persistent is chemia symptoms are already an established indicator for immediate angiography, independently of the ECG pattern; nevertheless, in the presence of ambiguous symptoms, this new recommendation may increase the use of emergency angiography.7
Oxygen therapy remains restricted to patients with arterial oxygen saturation < 90% or oxygen partial pressure < 60mmHg, and its routine use is not recommended for patients with oxygen saturation ≥ 90%. Previous guidelines recommended oxygen therapy for patients with oxygen saturation < 95%, breathing difficulties, or heart failure. The change in the new guidelines is consistent with the lack of evidence for a benefit from routine oxygen therapy in STEMI patients.8 The recommendation for opioid therapy is now less absolute, due to possible interference with the uptake of oral antiplatelet agents.
Cardiac ArrestIn patients in cardiac arrest but no ST-segment elevation, the decision to perform urgent coronary angiography should be taken after appropriate investigations to exclude noncoronary causes. This decision should also take account of the patient's neurological prognosis, and the guidelines specify the factors linked to a poor neurological outcome that would warn against an invasive coronary strategy. These contraindications include unwitnessed cardiac arrest, late arrival of the prehospital team, an initial nonshockable rhythm, and prolonged (> 20min) advanced life support without return to spontaneous circulation.
The guidelines do not recommend prehospital cooling by rapid infusion of cold fluids (class III, level of evidence B).9 A recent controlled trial did not demonstrate any benefit of therapeutic hypothermia (33°C) compared with normal body temperature (36°C).10 The new guidelines therefore adopt a neutral stance on this question, leaving targeted temperature management between 32°C and 36°C as an option so long as it does not delay PCI. Rigorous temperature management is recommended for all patients who remain unconscious after resuscitation from cardiac arrest (class I, level of evidence B). Debate is ongoing about the optimum target temperature, but clinical hypothermia should be avoided at all times.
STEMI Care NetworksSTEMI patient management should take place in centers forming part of a regional network. Centers offering primary PCI should provide this service 24hours a day and 7 days a week. Patients should be taken directly to the catheterization laboratory, avoiding the emergency department and hospitals with no PCI facility. In addition, key times to reperfusion should be recorded and reviewed to ensure the achievement and maintenance of quality standards. These new guidelines signal a renewed push to maintain and improve regional STEMI care networks and, where none exist, to establish new ones. The goal is to promote early and effective reperfusion and to ensure that as many patients as possible benefit from primary PCI. It is important that the networks established in Spain review and adapt their operational procedures to incorporate the new recommendations.
A new section called “Assessment of Quality of Care” evaluates clinical performance from a set of 16 quality indicators. These include measures of organization, reperfusion, risk evaluation, in-hospital and postdischarge treatment, and prognosis. The recent introduction of multiple primary PCI programs in Spain has not been accompanied by registries analyzing these indicators. Monitoring quality indicators in the different programs is essential to determine their quality and suitability.
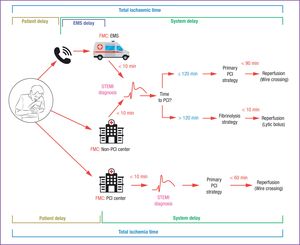
Choice of Reperfusion StrategyThe new guidelines include an excellent table with precise definitions of terms related to reperfusion strategy, providing welcome clarification of hitherto confusing terminology. A notable example is First Medical Contact (FMC), defined as the time point when a patient is first attended by a physician or other trained emergency health care specialist able to obtain and interpret an ECG and perform any necessary initial interventions, such as defibrillation. The FMC can be in an out-of-hospital setting (during ambulance transit) or in-hospital, when the patient arrives at the hospital by his or her own means; however, the FMC cannot be established through telephone contact because in this setting there is no possibility of medical intervention. An important advance in the new guidelines is the clarification of time zero in the reperfusion strategy clock; this is now defined as the moment when an ECG (obtained in a suitable clinical setting) indicates ST-segment elevation. Previously, the reperfusion strategy was based on the calculation of time elapsed since an imprecisely defined first medical contact.
The new guidelines recommend an ECG < 10minutes after the FMC and establish the time of STEMI diagnosis as the essential criterion for deciding on the reperfusion strategy. Primary PCI is recommended if wire crossing for reperfusion with PCI is feasible in ≤ 120minutes (with the performance qualtity indicator set at < 90min). When primary PCI is not possible within this time frame, fibrinolysis should be performed in < 10minutes, followed by emergency transfer to a PCI-capable center. When the FMC is at a PCI-capable hospital, the time limit for reperfusion is 60minutes (Figure).
Modes of patient presentation, components of ischemia time, and flowchart for reperfusion strategy selection. EMS, emergency medical system; FMC, first medical contact; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction. Reproduced from Ibáñez et al.2 with permission of OUP on behalf of the ESC.
The reperfusion strategy (primary PCI or fibrinolysis) is based on the time elapsed since symptom onset and on the estimated time needed to complete primary PCI after STEMI diagnosis:
- 1)
During the first 12hours after symptom onset, the decision is based on the estimated time to reperfusion by PCI. Immediately after a STEMI diagnosis, the competent emergency medical system personnel should estimate the time needed to achieve reperfusion by primary PCI (defined as wire crossing of the culprit lesion). If the time estimate is < 120minutes, the recommended procedure is primary PCI. If the calculated time from STEMI diagnosis to wire crossing is > 120minutes, a fibrinolytic bolus should be administered as soon as possible (target < 10minutes). This maximum target delay from STEMI diagnosis to fibrinolysis is shorter than in the previous guidelines, which proposed a maximum delay of 30minutes from FMC to fibrinolytic bolus administration. This change is a consequence of the improved definition of time zero in the reperfusion strategy clock (now set as the time of STEMI diagnosis) and the data from the STREAM clinical trial, in which the mean delay from randomization to fibrinolytic bolus administration was just 9minutes.11 Moreover, the new CPG document does not differentiate between patients with symptoms of short or long duration: if lesion wire crossing is believed to be achievable in < 120minutes, primary PCI is recommended even in patients seeking medical attention very early during the progress of the infarction.
- 2)
When the STEMI diagnosis is established more than 12hours after symptom onset, fibrinolysis is not recommended, and PCI should be considered in the following 2 circumstances: a) when the patient has hemodynamic or electrical instability in the presence of ongoing ischemic symptoms (class I, level of evidence C); and b) when the patient is attended between 12hours and 48hours after symptom onset (class IIa, level of evidence B). The indication of PCI up to 48hours is based on the results of the BRAVE-2 trial12 and is an important change from the previous guidelines, which did not recommend PCI in patients without persistent symptoms beyond 24hours after symptom onset.
- 3)
In stable patients with no persistent symptoms > 48hours after symptom onset, angiography is required to characterize the coronary anatomy, but there is no recommendation for routine opening of the culprit artery (class III, level of evidence A).
The new CPG document presents very clear and concise recommendations to facilitate rapid decision making about the reperfusion strategy. For example, the guidelines resolve the confusion in the previous version caused by the recommendation to perform reperfusion within 60minutes in patients in the very early stages of AMI (symptom duration ≤ 2hours). The new guidelines clarify that the goal is simply to perform primary PCI as soon as possible, with a maximum target delay of 90minutes as a quality indicator in all settings. Thus, fibrinolysis should be performed only when the calculated time from STEMI diagnosis to wire crossing is > 120minutes; fibrinolysis is never indicated for shorter intervals, even when STEMI diagnosis is very early. The new guidelines also sensibly eliminate the concept of door-to-balloon time, since this parameter did not distinguish between arrival at the catheterization laboratory or at the emergency room. The new guidelines also dispense with the concept of door-to-door time.
The simplification of reperfusion strategy selection is a very positive development. However, when in the midst of deciding between one or other strategy, it would help to have a more detailed evaluation of the implications of total ischemia time, the critical determinant of infarct size.13 This consideration makes it difficult to view fibrinolysis as preferable to primary PCI in patients with symptoms lasting many hours, even when PCI cannot be performed within 120minutes after FMC. On the other hand, a 120- minute delay to PCI could be excessive in high-risk patients seeking medical assistance within the first hour after symptom onset. It would also have been helpful if the new guidelines had mentioned (as did the previous version) that reducing the time to PCI is of paramount importance in patients presenting very early and that the concept of aborted infarction is no longer in use.
Primary PCI: Procedural and Pharmacological ConsiderationsThe most significant changes here are a strengthening of the recommendation for radial access (class 1, level of evidence A; previously class IIa, level of evidence B) and a recommendation to implant new-generation metal drug-eluting stents in all patients (class I, level of evidence A; previously class IIa, level of evidence A). Indeed, drug-eluting stent implantation is recommended even when dual antiplatelet therapy (DAPT) will likely be curtailed due to scheduled surgery or biopsies, etc. These changes are based on the results of long-term comparative studies showing that second-generation drug-eluting stents are more effective and safer than bare-metal stents.14 Moreover, a recent Spanish cost study in STEMI patients found similar cost-effectiveness for drug-eluting stents and their bare-metal counterparts.15 Therefore, there is also now no economic justification for not using drug-eluting stents in this setting.
Also worth special mention is the relegation of the recommendation for routine thrombus aspiration during primary PCI (previously class IIa, level of evidence B, now class III, level of evidence A). This change is based on the results of 2 large clinical trials with a total more than 17 000 patients; neither study found any benefit from routine thrombus aspiration, and 1 reported an increase in stroke with this procedure. Nevertheless, thrombus aspiration is firmly established in the interventional cardiologist community. Although its routine use is now contraindicated, the new guidelines leave the door open to its restricted use (class IIb) in patients with a large residual thrombus burden after vessel opening by PCI. However, this issue remains controversial because no trial has shown a benefit of thrombus aspiration in any patient subgroup. Another innovation in the new guidelines is the advice against routine delay of stenting after vessel opening with a guide wire or a balloon (class III, level of evidence B). The suggestion that delayed stenting might reduce the rate of acute complications has not been confirmed in specific studies.
The new guidelines also provide welcome detail on the appropriate approach to significant coronary stenosis in nonculprit vessels. Based on the results of 4 clinical trials, the guidelines advise that patients be considered for PCI in noninfarct-related arteries before hospital discharge (class IIa, level of evidence A); this procedure can even be performed during the primary PCI if the operator considers it appropriate. This is a significant change from the previous guidelines, which advised against routine complete revascularization. In the treatment of these severe lesions in nonculprit vessels, the guideliness propose revascularization during admission since that is the time used in all clinical trials. However, it seems reasonable to suppose that the same clinical outcome would be achieved by elective PCI of lesions in nonculprit vessels some weeks after hospital discharge. The presence of severe lesions in nonculprit vessels is of particular concern in STEMI patients with cardiogenic shock. In these patients, revascularization of all severe lesions should always be considered during the initial procedure. Nevertheless, since the publication of this guideline, the CULPRIT-SHOCK16 has questioned the strategy of complete revascularization on finding an association with higher mortality.
Significant changes have also been introduced concerning the recommended antigoagulant drugs used during primary PCI. The strongest recommendation is for unfractionated heparin (class I, level of evidence C), with enoxaparin and bivalirudin proposed as less attractive alternatives (class IIa, level of evidence A). These changes represent a strengthening of the recommendation for enoxaparin (previously class IIb, level of evidence B) but a weakening of that for bivalirudin (previously class I, level of evidence B). Oral antiplatelet therapy recommendations are similar to those in the previous guidelines, with the strong P2Y12 inhibitors prasugrel and ticagrelor as the first-line therapy and clopidogrel reserved for when these first 2 are contraindicated or unavailable. A new recommendation is the possibility to use the intravenous P2Y12 inhibitor cangrelor when the patient has not previously received oral therapy with prasugrel, ticagrelor, or clopidogrel (class IIb, level of evidence A). This could be an interesting option for specific patient subgroups (intubated patients). Glycoprotein IIb/IIIa inhibitors should be considered only as bailout therapy in the event of reperfusion failure (no-reflow) or thrombotic complications (class IIa, level of evidence C). The new guidelines eliminate the previous recommendation to consider this procedure in at-risk patients during transfer to the primary PCI center.
FibrinolysisWhen the selected reperfusion strategy is fibrinolysis, the fibrinolytic bolus should be injected < 10minutes after STEMI diagnosis, and the patient should be transferred immediately to a primary PCI center. The reduction in the recommended time limit to 10minutes is reasonable given the ease of delivering current fibrinolytic agents by intravenous bolus injection.11 If there is no evidence of reperfusion 60 to 90minutes after bolus injection, rescue PCI must be performed immediately. If fibrinolysis appears to have been successful, the guidelines indicate coronary angiography, with PCI of the infarction-related artery if appropriate, between 2 and 24hours after fibrinolytic bolus injection (class I, level of evidence A, compared with the previous range of 3 to 24hours, in class IIa, level of evidence A). Bringing forward the timing of angiography after successful fibrinolysis is a very practical measure that will allow most patients to be scanned immediately on arrival at hospital. This will likely be the best option when the catheterization team is present (during normal working hours), whereas at other times angiography can be scheduled for the next day.
As in previous versions, the new guidelines recommend the use of specific fibrinolytic agents. The indicated antithrombotic co-therapy in this setting is a combination of aspirin, clopidogrel (loading dose 300mg, or 75mg in patients ≥ 75 years old), and enoxaparin (intravenous followed by subcutaneous). The new guidelines recommend halving the dose of tenecteplase in patients ≥ 75 years old (class IIa, level of evidence B), based on the results of the STREAM trial, in which switching to the lower dose reduced intracranial bleeding.11 The reduced tenecteplase dose removes the need for the clopidogrel loading dose, and will likely result in lower rates of cerebral hemorrhage in elderly patients. Another new feature is the option to switch maintenance therapy from clopidogrel to prasugrel, after a minimum 48-hour delay, in patients treated according to a pharmacoinvasive strategy (class IIb, level of evidence C).
Approach to Heart Failure and Arrhythmias in AMIThe guidelines provide a detailed description of the management of patients with varying degrees of heart failure due to ventricular dysfunction. The guideline authors have simplified the table summarizing recommendations for the management of STEMI complicated by heart failure, and there is no stratification by Killip class. The recommendation for opioid therapy in patients with pumonary edema and severe dyspnea has been relegated to class IIb, level of evidence C (previously IC). Moreover, the guidelines no longer recommend intravenous calcium antagonists to control frequency in atrial fibrillation (AF), and the use of intravenous beta-blockers for this purpose has been relegated from class I, level of evidence A to class I, level of evidence C. Aldosterone receptor antagonists are recommended in patients with heart failure and ejection fraction ≤ 40%; however, the corresponding recommendations table refers to acute, subacute, and long-term care. The guidelines thus might have benefitted from more detail on the use of aldosterone receptor antagonists.
The use of inotropic therapy or vasopressors in patients with cardiogenic shock has dropped from class IIa in the previous version to class IIb in the new guidelines (level of evidence C in both cases). Similarly, the recommendation for ultrafiltration in these patients has dropped from class IIa to class IIb (level of evidence B in both cases). The approach to mechanical complications in AMI should be decided by the multidisciplinary team and implemented as soon as possible (class I, level of evidence C). As mentioned above, patients with cardiogenic shock should undergo complete coronary revascularization (class IIa, level of evidence C). However, intra-aortic balloon pumping should be limited to patients with hemodynamic instability or cardiogenic shock due to mechanical complications (class IIa, level of evidence C) and is not recommended as a routine treatment for patients with cardiogenic shock due to ventricular dysfunction (class III, level of evidence B).
The recommended approach to arrhythmic complications is in line with the corresponding ESC guidelines. Implantable cardioverter defibrillator therapy is recommended to prevent sudden cardiac death in patients in New York Heart Association class II-III and with left ventricular ejection fraction ≤ 35% at ≥ 6 weeks post-AMI; however, this recommendation is restricted to patients who are expected to survive for at least 1 year with good functional status. Implantable cardioverter defibrillator implantation or temporary use of a wearable cardioverter defibrillator can also be considered <40 days after AMI in selected patients at risk of sudden arrhythmic death (class IIb, level of evidence C).
Management During Hospitalization and at DischargeThe new guidelines introduce few changes to the initial in-hospital management of STEMI patients after reperfusion. There is now a clearer definition of patients at arrhythmic risk who should be considered for prolonged ECG monitoring (> 24hours); the key patient characteristics are hemodynamic instability, the presence of severe arrhythmia, ejection fraction < 40%, failed reperfusion, untreated critical coronary stenosis in nonculprit vessels, or PCI-related complications. There is now a class IIa, level of evidence C recommendation (previously class IIb) to transfer stable patients to a referral hospital without PCI capacity on the same day as successful revascularization. Early discharge (48-72hours) is suggested for low-risk patients (< 70 years old, ejection fraction > 45%, disease affecting 1 or 2 vessels, successful PCI, and no persistent arrhythmias). This is consistent with the recommendation for early discharge (48-72hours) of low-risk patients when provision can be made for early rehabilitation and appropriate follow-up.16 The guidelines thus strengthen the recommendation for early discharge (48-72hours) of low-risk patients (class IIa) with respect to the earlier recommendation for discharge at 72hours (class IIb).
A major innovation in the new guidelines is the description of specific patient subgroups that were poorly defined in the previous version:
- 1)
Myocardial infarction with nonobstructive coronary arteries (MINOCA). The new guidelines include a new section on STEMI patients showing no significant coronary stenosis on emergency angiography. This section details possible etiologies (myocarditis, myocardial disorders, epicardial and microvascular involvement, pulmonary embolism, and imbalance between oxygen supply and demand) and recommends complementary investigations to identify the underlying cause.
- 2)
Patients taking oral anticoagulation. Oral anticoagulant therapy is increasingly frequent among patients attending Spanish hospitals, above all those with nonvalvular AF, and the new ESC guidelines review their acute and chronic management. The principal recommendations are as follows: a) primary PCI reperfusion to avoid the risk of hemorrhage with fibrinolysis; b) parenteral anticoagulant therapy independently of the last anticoagulant dose; c) antiplatelet therapy with aspirin and clopidogrel (avoiding prasugrel, ticagrelor, and glycoprotein Ib/IIIa inhibitors); d) maintenance of triple antithrombotic therapy (combining an oral anticoagulant, aspirin, and clopidogrel) for 6 months. However, safety concerns raised by the recent PIONEER18 and RE-DUAL-PCI19 studies indicate that this last recommendation should be weighed against the option of dual therapy with an antiplatelet agent and a direct acting anticoagulant (rivaroxaban or dabigatran).
- 3)
Elderly patients: The guidelines emphasize the difficulty of STEMI diagnosis in elderly patients due to the high frequency of atypical symptoms and the risk of hemorrhage linked to antithrombotic therapy. However, no specific recommendations are made for frail elderly patients or those with comorbidities, 2 subgroups that present difficulties in decision making about STEMI treatment strategies.
- 4)
Patients with renal dysfunction: The guidelines recommend early estimation of glomerular filtration rate, maintenance of adequate hydration, use of the minimum amount of contrast agent during PCI, and adjustment of antithrombotic therapy in relation to the patient's age and glomerular filtration rate. These dose adjustments can be facilitated with the STEMI CPG mobile application, which calculates doses as a function of the grade of renal dysfunction.
- 5)
Patients with diabetes. The choice of antithrombotic therapy and reperfusion strategy should not differ between patients with and without diabetes. The guidelines mention that randomized studies of patient subgroups have suggested a greater absolute risk reduction in patients with diabetes when treated with prasugrel or ticagrelor than when treated with clopidogrel. However, the association between diabetes and an increased benefit of prasugrel or ticagrelor was nonsignificant, suggesting that any benefit would occur equally in patients without diabetes. The guidelines also highlight the importance of adequate glucose control, with particular emphasis on avoiding hypoglycemia. In patients on metformin or SGLT2 inhibitors, the guidelines recommend monitoring of renal function for 3 days after PCI.
For risk stratification, the guidelines stress the importance of clinical variables (especially the GRACE score) and imaging analysis. Echocardiography is a routine procedure after primary PCI. However, superior evaluations of myocardial viability and residual ischemia are obtained with other imaging techniques, such as single-photon emission computed tomography (SPECT), cardiac magnetic resonance (CMR), and positron emission tomography (PET). Therefore these techniques, in particular CMR, are now a class IIa recommendation (previously class IIb) when echocardiography is considered inconclusive or of poor quality. This has important implications for patients with multivessel disease in whom stenosis revascularization in nonculprit vessels has been delayed for a subsequent intervention, rather than being performed at the time of primary PCI. In this situation, if lesion severity has not been evaluated by fractional flow reserve, a decision guided by an accurate ischemia test might be more advisable than performing complete revascularization guided by the oculostenotic reflex. However, it is has to be recognized that many Spanish hospitals do not have the economic or material resources to support imaging techniques other than echocardiography, placing severe limits on their general adoption.
Postdischarge and Long-term TreatmentThe new guidelines strengthen the level of evidence for cardiac rehabilitation and smoking cessation interventions (including pharmacological interventions with nicotine replacement therapy, varenicline, or bupropion); these interventions are both class I recommendations but are underused in Spain. There are no changes in the recommendations for the main long-term pharmacological therapies: beta-blockers, angiotensin converting enzyme inhibitors, and aldosterone receptor antagonists. Despite doubts about the efficacy of chronic beta-blocker therapy in patients without ventricular dysfunction or heart failure, the level of recommendation has been maintained. In contrast, the guidelines strengthen the recommendation (class I, level of evidence B) for gastric protection with a proton pump inhibitor in patients with risk factors for gastrointestinal bleeding. The updated guidelines on lipid-lowering therapy retain the recommendation for early lipid profiling (now with no need for fasting, which simplifies the logistics) and prompt initiation of high-intensity statin therapy. There are now specific therapeutic recommendations for the long-term control of low-density lipoprotein cholesterol (LDL-C), in line with the recommendations in the ESC guidelines on cardiovascular prevention. The specific target is LDL-C < 70mg/dL or a reduction of > 50% if baseline LDL-C is between 70mg/dL and 135mg/dL (class I, level of evidence B). Based on the results of previous studies, the new guidelines recommend concomitant therapy with ezetimibe or anti-PCSK9 antibody in high-risk patients with LDL-C ≥ 70mg/dL despite statin therapy at the highest tolerated dose (class IIa, level of evidence A). However, no specific guidance is offered on which drug should be used, when it should be administered, or to which patients.
Lastly, changes have been made to several recommendations related to long-term antithrombotic therapy, bringing them in line with the new ESC guidelines on DAPT.20 Routine DAPT is still recommended for 12 months, with the additional recommendation that this therapy be considered for patients not treated by PCI (class IIa, level of evidence C); with this change, stenting is no longer the only criterion for establishing DAPT duration. In contrast, discontinuation of DAPT after 6 months is recommended for patients at high risk of bleeding (class IIa, level of evidence B). Moreover, in patients at high ischemic risk who have had no bleeding events in the first 12 months post-AMI, continuation of ticagrelor therapy (60mg/ 12h) can be considered up to a maximum of 3 years (class IIb, level of evidence B). In line with previous versions, the new ESC guidelines recommend a polypill strategy to improve treatment adherence (class IIb, level of evidence B).
Given the complexity of oral anticoagulation therapy, it is interesting to see the focus in the guidelines on patients requiring this treatment. The recommendation for simultaneous antiplatelet therapy is maintained (class I, level of evidence C), with initial triple therapy for 1 to 6 months according to the balance between coronary and bleeding risk (class IIa, level of evidence C). However, ticagrelor and prasugrel should be avoided in triple therapy (class III, level of evidence C). The guidelines introduce 2 new recommendations for de novo anticoagulation in STEMI patients. Detection of a left ventricular thrombus is an indication for anticoagulation therapy for up to 6 months, with monitoring by imaging (class IIa, level of evidence C). The second new recommendation is AF in the acute phase of AMI, which is an indication for long-term anticoagulation, in accordance with the CHA2DS2-VASc score (class IIa, level of evidence C). This recommendation is based on the evidence that self-limiting AF episodes in the acute phase of STEMI are associated with a higher incidence of stroke during follow-up.
CONFLICTS OF INTERESTE. Abu -Assi is an Associate Editor on Rev Esp Cardiol; E. López de Sá receives consultancy fees from Servier, Pfizer, and Novartis.
SEC Working Group for the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-segment Elevation, Expert Reviewers for the 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-segment Elevation, and the SEC Guidelines Committee.
The names of all authors of this article are listed in the Appendix.