Patients who have undergone angioplasty with stenting can be reintegrated into normal life at an early stage, thanks to the absence of sequelae associated with the procedure itself. Consequently, these patients can be involved earlier in the second stage of cardiac rehabilitation. Although rehabilitation for coronary patients follows the general guidelines used for all patients, which were developed with the secondary prevention of coronary artery atherosclerosis in mind, the specific form of rehabilitation adopted for each individual with ischemic heart disease will depend on the patient's circumstances, including the revascularization technique used. Regular physical exercise (i.e. physical training), in itself, has substantial cardiovascular benefits for both primary and secondary cardiovascular prevention. In patients who have had a myocardial infarction, training decreases mortality, increases functional capacity and improves ventricular function and remodeling. It is also thought to boost the collateral circulation. In addition, training improves endothelial function and stimulates the circulation of stem cells. It has been shown that physical training after percutaneous revascularization decreases the number of cardiac events. Moreover, in patients with stable angina, it results in fewer events than percutaneous revascularization.
Keywords
In line with the clinical practice guidelines policy of the Spanish Society of Cardiology (SEC),1 this article presents the novel, pertinent, and contentious aspects of the 2018 update on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC), developed in conjunction with the International Society of Gender Medicine (IGM), the German Institute of Gender in Medicine (DGesGM), the European Society of Anaesthesiology (ESA), and the European Society of Gynecology (ESG).2
At the suggestion of the SEC Guidelines Committee and the coordinators assigned to this document, a group of expert cardiologists and gynecologists was selected to review the guidelines. The objective was to comment on the nature and timeliness of these recommendations, analyze the methodology, and highlight the novelties (Table) and positive, questionable, or omitted aspects (Table 1). These evaluations were used to develop a joint document, which was further reviewed by cardiologists appointed by the SEC Sections of Clinical Cardiology, Cardiovascular Risk and Cardiac Rehabilitation, and Pediatric Cardiology and by the Spanish Society of Gynecology and Obstetrics (SEGO).
Notable Novelties of the 2018 Guidelines
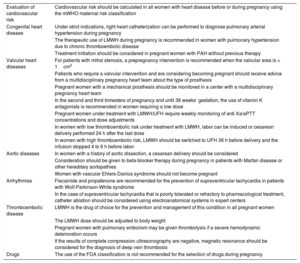
| Evaluation of cardiovascular risk | Cardiovascular risk should be calculated in all women with heart disease before or during pregnancy using the mWHO maternal risk classification |
| Congenital heart disease | Under strict indications, right heart catheterization can be performed to diagnose pulmonary arterial hypertension during pregnancy |
| The therapeutic use of LMWH during pregnancy is recommended in women with pulmonary hypertension due to chronic thromboembolic disease | |
| Treatment initiation should be considered in pregnant women with PAH without previous therapy | |
| Valvular heart diseases | For patients with mitral stenosis, a prepregnancy intervention is recommended when the valvular area is < 1cm2 |
| Patients who require a valvular intervention and are considering becoming pregnant should receive advice from a multidisciplinary pregnancy heart team about the type of prosthesis | |
| Pregnant women with a mechanical prosthesis should be monitored in a center with a multidisciplinary pregnancy heart team | |
| In the second and third trimesters of pregnancy and until 36 weeks’ gestation, the use of vitamin K antagonists is recommended in women requiring a low dose | |
| Pregnant women under treatment with LMWH/UFH require weekly monitoring of anti-Xa/aPTT concentrations and dose adjustments | |
| In women with low thromboembolic risk under treatment with LMWH, labor can be induced or cesarean delivery performed 24 h after the last dose | |
| In women with high thromboembolic risk, LMWH should be switched to UFH 36 h before delivery and the infusion stopped 4 to 6 h before labor | |
| Aortic diseases | In women with a history of aortic dissection, a cesarean delivery should be considered |
| Consideration should be given to beta-blocker therapy during pregnancy in patients with Marfan disease or other hereditary aortopathies | |
| Women with vascular Ehlers-Danlos syndrome should not become pregnant | |
| Arrhythmias | Flecainide and propafenone are recommended for the prevention of supraventricular tachycardia in patients with Wolf-Parkinson-White syndrome |
| In the case of supraventricular tachycardia that is poorly tolerated or refractory to pharmacological treatment, catheter ablation should be considered using electroanatomical systems in expert centers | |
| Thromboembolic disease | LMWH is the drug of choice for the prevention and management of this condition in all pregnant women |
| The LMWH dose should be adjusted to body weight | |
| Pregnant women with pulmonary embolism may be given thrombolysis if a severe hemodynamic deterioration occurs | |
| If the results of complete compression ultrasonography are negative, magnetic resonance should be considered for the diagnosis of deep vein thrombosis | |
| Drugs | The use of the FDA classification is not recommended for the selection of drugs during pregnancy |
anti-Xa/aPTT, factor Xa inhibitors/activated partial thromboplastin time; FDA, Food and Drug Administration; LMWH, low-molecular-weight heparin; mWHO, modified World Health Organization classification; PAH, pulmonary arterial hypertension; UFH, unfractionated heparin.
The new version of the ESC guidelines is the result of a systematic review of the literature published between 2011 and 2016 and is an updated tool that helps physicians to manage pregnant women with heart disease. The preamble stresses that the recommendations are designed to support health care professionals, because they are the ones who are ultimately responsible for clinical decision-making and who must manage the complex balance between 2 often-times opposing therapeutic strategies: one aims to optimize maternal health outcomes, whereas the other aims to ensure adequate fetal development by minimizing the risks of possible maternal treatments, including the consequences of prematurity.
As a novelty, the format is more functional and new sections have been created, such as recommendation tables summarizing the fundamental and novel aspects with respect to previous editions, ordered according to their level of evidence. The standard levels of evidence (A, B, and C) and recommendations (classes I, IIa, IIb, and III) are used.
2GENERAL CONSIDERATIONS2.1Physiological adaptations to pregnancyThe section on maternal adaptation to pregnancy has been reduced vs previous editions and, although not explicitly mentioned in the guidelines, it should be remembered that the puerperium is a period of special vulnerability for this population.
2.2Prepregnancy counselingEven more than in the previous version, this new edition emphasizes the importance of preconception counseling for all women with heart disease before pregnancy is planned (I C).3 As a novel and pertinent aspect, the guidelines believe that this advice should be delivered via a multidisciplinary approach. Accordingly, the concept of a specialized multidisciplinary team, the pregnancy heart team, is presented (I C). This group of experts, comprising at least 1 cardiologist, obstetrician, and anesthesiologist, should jointly perform the preconception counseling, monitor the progress of the pregnancy, and guide decision-making regarding delivery and puerperium.
The previous editions included different risk estimation scales for maternal complications and offering pregestational advice while stressing the modified WHO classification (mWHO). The new guidelines consider the mWHO to be the risk scale of choice (I C) because it better predicts maternal risk vs other scales, as shown in registries4 and specifically in Spain.5 The guidelines include an estimate, in each of the 5 categories, of the probability of an adverse cardiac event and suggest the frequency of follow-up during pregnancy and the type of center that should perform such monitoring or handle the delivery according to category (see Figure 3 of the guidelines). As a summary of the other published risk scales used to estimate both maternal and fetal risk, the new document also includes a table with a set of variables that can help to predict events, such as history of smoking or natriuretic peptide (NT-proBNP) elevation above 128 pg/mL at 20 weeks’ gestation.
2.3Cardiovascular diagnosis in pregnancyThe guidelines continue to emphasize that the interpretation of clinical signs is more difficult in pregnant patients than in nonpregnant ones and continues to recommend the usual diagnostic tests (electrocardiography, echocardiography, and exercise testing) to assess risk in women with heart disease who wish to conceive. In terms of ionizing radiation, the principle of “as low as reasonably achievable” is maintained and a radial approach with abdominal protection is preferable if cardiac catheterization is required.
2.4Fetal assessmentRegarding the fetal evaluation, the guidelines reiterate the growing importance of 12-week obstetric ultrasound, whose sensitivity and specificity for major congenital heart diseases are as high as 85% and 99%, respectively. Indeed, a fetus with a normal measurement of nuchal translucency has a low risk of congenital heart disease (about 1/1000). The guidelines emphasize that this examination should ideally be performed (or corroborated if it has already been done) between weeks 19 and 22 (I C), when it shows the highest diagnostic yield.
2.5Interventions in the mother during pregnancyGeneral considerations also include indications for cardiac surgery or percutaneous treatment during pregnancy when necessary due to failure of medical treatment or because the mother's life is in danger (IIb C). Both cardiac surgery and percutaneous treatments should not be performed until the second trimester of pregnancy and, given the high fetal mortality, a presurgical cesarean delivery is recommended after week 26 (IIa C).
2.6Timing and mode of deliveryThe benefit of vaginal delivery is recognized (I C), and an assisted delivery involving forceps or a ventouse can be considered to reduce maternal effort and shorten the expulsive phase. The cardiac indications for elective cesarean delivery of the previous edition are maintained (IIa C): severe heart failure, oral anticoagulant use at the time of labor, significant dilatation of the ascending aorta, severe aortic stenosis, and Eisenmenger syndrome.
A novelty of these guidelines is that, based on the meta-analysis of Mishanina et al.,6 labor induction should be considered after week 40 for all women with cardiac disease (IIa C); this is not the standard approach in Spain. That meta-analysis included data on more than 31 000 women from the general population and identified a 50% reduction in fetal mortality among pregnant women with induced labor vs those with noninduced labor and a 12% reduction in the rate of emergency cesarean deliveries, although the benefits were not stratified by gestational age. However, there is no evidence in the literature supporting systematic labor induction for women with cardiac disease with good cardiac function, particularly when a spontaneous labor is both possible and expected. Therefore, for these pregnant women, the standard induction strategy can be considered once week 41 has been reached, as long as the women have been informed of the advantages and disadvantages of labor induction. The current guidelines are less explicit than previous editions in terms of specifying when to end a pregnancy in the presence of fetal growth restriction, given that each day that the pregnancy is prolonged between weeks 24 and 28 increases the probability of fetal survival without sequelae by 1%, and the probability increases to 2% between weeks 29 and 32.
Another novelty is in the reference to the drugs used for labor induction. Although the guidelines again mention the theoretical risk of coronary vasospasm, the document considers both misoprostol and dinoprostone to be safe at their usual doses. The infusion of 2 IU of oxytocin after delivery is still recommended, followed by an infusion of 12 MIU/min for 4hours to reduce the risk of postpartum bleeding; however, ergometrine and prostaglandin F2 alpha should be avoided. Antibiotic prophylaxis for endocarditis is not advised for vaginal or cesarean deliveries.
2.7Methods of contraception and termination of pregnancy, and in vitro fertilizationContraceptive advice has an important place in these new guidelines. Given to women with heart disease from menarche by gynecologists and experienced cardiologists, the advice aims to avoid the risks associated with an unplanned pregnancy. “Progestogen-only” contraceptives are a safe alternative to combined oral contraceptives, preparations that increase the risk of thromboembolic disease. Emergency contraception is safe with all types of heart disease and new intrauterine devices with levonorgestrel, which are smaller and easier to insert, are indicated for women with menorrhagia. The guidelines mention the advantage of hysteroscopic tubal sterilization over laparoscopic sterilization, although the approach is not without controversy, given the possible and unconfirmed association with chronic pelvic pain.
Finally, general considerations regarding assisted reproduction for patients with heart disease are added to the guidelines, particularly the need for low-dose ovarian stimulation and, above all, single-embryo transfer. Fertility treatments are contraindicated in women in mWHO class IV and should be considered with caution by women in mWHO III or on anticoagulant therapy.
3CONGENITAL HEART DISEASE AND PULMONARY HYPERTENSION3.1Pulmonary hypertension and Eisenmenger syndromePregnancy is still discouraged in this group of patients (III); this is the only recommendation with a b level of evidence. The risk stratification is the same as that of nonpregnant patients and the guidelines recommend that patients without previous pulmonary hypertension therapy begin combination therapies (IIa C), starting with oral sildenafil; endothelin antagonists are contraindicated. The use of sildenafil should be assessed with caution in patients with placental insufficiency, given the increased rate of intrauterine mortality found in a recent study of fetuses with fetal growth restriction.7
In patients on previous pulmonary hypertension therapy, intravenous treatments should be continued, although the withdrawal of embryotoxic drugs should be appraised (IIa C). Anticoagulation with low−molecular-weight heparin should be considered, especially in patients with chronic thromboembolic disease (I C). The follow-up should be very close and conducted in specialized centers, even weekly in the last weeks of pregnancy, with assessment of saturation and right ventricular function at each visit. Local anesthesia is recommended over general and a detailed delivery plan should be devised, with therapy maintenance in the puerperium. In patients with Eisenmenger syndrome, anticoagulation should be used with caution due to alterations in coagulation factors or thrombocytopenia.
3.2Congenital heart diseaseThere are few changes to these recommendations, which are still based on expert opinion. The indications for prepregnancy interventions are generally not different for women who are considering becoming pregnant than for other patients and an experienced fetal cardiologist should still perform fetal echocardiography in weeks 19 to 22 (I C).
Atrial septal defect: closure is still not recommended to prevent paradoxical embolism, and the guidelines advise nonpharmacological prevention of venous stasis and the use of air filters.
Ventricular septal defect: 1 or 2 examinations are recommended during pregnancy to rule out pulmonary hypertension.
Atrioventricular septal defect: in these guidelines, this condition is considered class mWHO II-III due to the risk of arrhythmias and increase in atrioventricular valve regurgitation and heart failure.
Coarctation of the aorta: pregnancy is discouraged in women with severe recoarctation and its treatment with a covered stent is only recommended if there is refractory hypertension or maternal or fetal risk.
Pulmonary valve and right ventricular outflow tract disease: unlike previous editions, the current guidelines do not consider that these entities increase obstetric risk. A percutaneous valvuloplasty should be considered in patients with severe symptomatic stenosis despite treatment.
Tetralogy of Fallot: maternal screening for 22q11 deletion is recommended in genetic counseling (as should be done for all pregnant women with a conotruncal heart disease). If the abnormality has been well repaired, the patient is considered to be in class mWHO II and to require follow-up every trimester; monitoring should be more frequent in patients with severe pulmonary regurgitation. Early delivery or transcatheter valve implantation can be considered if patients fail to respond to conservative treatment.
Ebstein anomaly: pregnancy is only discouraged in symptomatic women with cyanosis with SatO2 < 85% or heart failure (IIa C). The possible occurrence of progressive cyanosis or paradoxical embolism should be monitored in women with interatrial shunt.
Transposition of the great arteries: in patients with an arterial switch, the risk seems low, but women with a dilated neoaorta should be monitored. Patients with a Mustard or Senning atrial switch or congenital corrected transposition are considered in class mWHO III and their ventricular function, symptoms, and heart rate should be monitored every 4 to 8 weeks. Pregnancy is discouraged in women with severe systemic tricuspid regurgitation, an advanced New York Heart Association functional class, or systemic ventricle dysfunction < 40% (IIa C).
Fontan circulation: these patients are at high risk (mWHO III or IV) due to the risk of arrhythmias and functional class deterioration and show a high incidence of miscarriage, fetal complications, and peripartum bleeding. Monthly follow-up is recommended, as well as, as a novelty, assessment of therapeutic anticoagulant therapy (IIa C), after consideration of the hemorrhagic risk. Pregnancy is discouraged in patients with a SatO2 < 85%, ventricular dysfunction, moderate or severe atrioventricular valve regurgitation, refractory arrhythmias, or protein-losing enteropathy (III C).
3.3Aortic diseasesThe guidelines do not introduce any pertinent developments in management or level of evidence, although they continue to promote the optimal treatment of these patients.
A complete study of the aorta is recommended, as well as multidisciplinary prepregnancy counseling for women with aortopathy or a bicuspid aortic valve. Pregnancy is discouraged in patients with vascular Ehlers-Danlos syndrome, hereditary aortic disease with a diameter > 45mm, bicuspid aortic valves > 50mm (> 27mm/m2), Turner syndrome with > 25mm/m2, and a history of dissection (III C). However, one critically important aspect that is overlooked involves recommendations on pregestational prophylactic surgery according to aortic diameter, suggesting that the general guidelines for aortic disease should be followed.
During pregnancy, ultrasound follow-up is recommended every 4 to 12 weeks until 6 months postpartum (I C). The indication for cesarean delivery is maintained when the aortic diameter is > 45mm or there is a history of dissection (IIa C); ergometrine is discouraged (III C). Due to the lack of new evidence, a more controversial aspect is that prophylactic surgery during pregnancy is now indicated when the aortic diameter is > 45mm (previously was > 50mm) and is increasing rapidly (IIa C); in addition, the rate of the increase is not defined. In the case of an essential operation, presurgical delivery should be considered if the fetus is viable (IIa C). As a general rule, beta-blocker therapy is recommended in hereditary aortopathies (IIa C) and celiprolol is specifically recommended in vascular Ehlers-Danlos syndrome (I C).
4VALVULAR HEART DISEASERegarding patients with mitral stenosis, the authors reiterate that pregnancy can trigger hemodynamic instability in previously asymptomatic patients.8 In addition, an intervention should be performed in patients with a mitral valve area < 1.0cm2 before gestation (I C) and should be considered when the area is < 1.5cm2 (IIa C), preferably percutaneously, if possible.
In aortic stenosis patients who are severely symptomatic and not amenable to balloon valvuloplasty, the new guidelines mention the novel alternative of transcatheter aortic valve implantation, despite the limited experience with this technique during pregnancy.
In pregnant women with mitral regurgitation, the focus is on those with moderate or severe regurgitation, due to increased risk of heart failure during pregnancy. Women with severe mitral regurgitation with symptoms or left ventricular dysfunction constitute the high-risk group.
5VALVULAR ATRIAL FIBRILLATION (NATIVE VALVES)Given the high thromboembolic risk associated with pregnancy, immediate anticoagulation is recommended with low−molecular-weight heparin (LMWH) at therapeutic doses–with no need to start with unfractionated heparin, as mentioned in the previous guidelines–, followed by a switch to oral anticoagulants in the second trimester, with INR control at the usual intervals. The contraindication is emphasized for pregnant women to the new nonvitamin K antagonist oral anticoagulants (OACs).
6PROSTHETIC VALVESThis section plays a larger role, the recommendation table is expanded, and flowcharts are added to promote the understanding of anticoagulant therapy during pregnancy.
The guidelines once again stress the role played by the multidisciplinary pregnancy heart team in the choice of the type of valvular prosthesis for women who desire to become pregnant (I C). Now a class IIa C recommendation, a biological prosthesis should be implanted in women who wish to become pregnant.
In women with a mechanical prosthesis, pregnancy is still considered high risk for both the woman and the fetus due to the anticoagulant therapy,9 and close monitoring is required of the INR, aPTT, or anti-Xa. Heparin therapy is noted to increase the risk of prosthetic thrombosis.
Regarding LMWH therapy, the optimal anti-Xa concentrations in this population are still unclear, as well as the importance of peak vs trough values. A new IIb recommendation indicates that the anti-Xa level could be measured before LMWH administration, in addition to 4 to 6hours after the treatment. It is worth noting that the different anticoagulation targets for LMWH and OACs depend on prosthesis location and type or the presence of added risk factors for thrombosis.
Flowcharts are provided to better illustrate the different therapeutic guidelines, which depend on whether low- or high-dose OACs are required for the target INR, and the guidelines stress that changes to the anticoagulation regimen should be implemented in a hospital setting (I C).
The authors emphasize that, in these patients, delivery should be planned and even recommend that the time of delivery be predicted to ensure a safe peripartum anticoagulation regimen (I C). Vaginal delivery is possible as long as the mother has previously been switched to intravenous heparin, and cesarean delivery is considered a good option, particularly for patients with a high risk of prosthetic thrombosis, to minimize the time without OACs. Cesarean delivery is also recommended if labor begins while the patient is under treatment with OACs or less than 2 weeks have passed since their interruption (I C).
7CORONARY ARTERY DISEASEMore space is devoted to coronary artery disease (CAD) than in the previous guidelines, and the authors note that CAD accounts for more than 20% of maternal cardiac deaths.9 For infarction, a multidisciplinary approach is recommended with intensivists, obstetricians, and cardiologists. Primary angioplasty is recommended, possibly with drug-eluting stents, for ST-segment elevation acute myocardial infarction. The recommendations now also include the possibility of invasive treatment of non−ST-segment elevation acute myocardial infarction in patients with high-risk criteria (IIa C). Aspirin and clopidogrel can be used (the latter, for as short as possible). The use of higher potency antiplatelet agents (prasugrel and ticagrelor) is not recommended.
8CARDIOMYOPATHIES AND HEART FAILUREThere are no changes in the management of patients with dilated or hypertrophic cardiomyopathy from previous guidelines. The only noteworthy aspect concerns bromocriptine treatment of peripartum cardiomyopathy, recommended for at least 1 week, or longer if the ejection fraction is < 25% (IIb B).9 A new pregnancy is discouraged if the ejection fraction does not recover. Heart failure treatment should be maintained for at least 6 months, even if ventricular function recovers.
9HEART FAILURE DURING AND AFTER PREGNANCYThis section has also gained relevance in these guidelines. The text is more extensive, with expansion of aspects related to management, devices, and heart transplant. In addition, flow charts are added to better illustrate the management of acute heart failure in pregnancy and in the postpartum period.
Patients with hemodynamic instability or shock should be urgently transferred to a unit specialized in acute cardiological care where mechanical circulatory support is available (IIa C). In these patients, a cesarean delivery should be considered. In the specific case of peripartum cardiomyopathy, levosimendan should preferentially be used as an inotropic agent due to the higher toxicity of beta-adrenergic agonists.
In the case of acute or subacute heart failure, the guidelines reiterate that fetotoxic drugs (angiotensin-converting enzyme inhibitors [ACEIs], angiotensin II receptor antagonists [ARA-IIs], neprilysin inhibitors, and mineralocorticoid receptor antagonists) should be avoided and that hydralazine and nitrates are preferred; the use of diuretics should be restricted to patients with pulmonary congestion and selective ?1-blockers should be used instead; they should be initiated with caution. The indications for anticoagulation are similar to those in nonpregnant individuals (eg, severe dysfunction, atrial fibrillation). Breastfeeding continues to be discouraged in patients with severe heart failure (IIb B).
10ARRHYTHMIASThis section contains a novel table of recommendations on the level of surveillance and the actions necessary during delivery according to risk of hemodynamic deterioration. There are no changes in pharmacological treatment. The guidelines still consider electrical cardioversion to be safe in patients with hemodynamic instability (I C). Catheter ablation of supraventricular arrhythmias becomes a IIa C recommendation9 and should preferably be performed after the second trimester. Pacemaker or defibrillator implantation can be safely performed from the 8th week (I C).
11HYPERTENSIVE DISORDERSHypertensive disorders continue to be one of the most frequent medical problems faced by mothers and the fetus (5%-10% of pregnancies). The definition of arterial hypertension (HT) in pregnancy remains systolic blood pressure (SBP) ≥ 140 or diastolic blood pressure (DBP) ≥ 90mmHg and, despite the lack of evidence, the indications remain for pharmacological treatment if the arterial pressure is ≥ 150/95mmHg or ≥ 140/90mmHg in the presence of associated risk factors. The risk-benefit of HT therapy10 is related to a lower incidence of severe maternal HT, pre-eclampsia, and fetal events, but the therapeutic targets still lack scientific evidence.
Regarding standard HT-related laboratory tests (hemoglobin, platelets, transaminases, renal function, and proteinuria), the guidelines note, for the first time, the predictive value of a sFlt-1:PlGF (placental growth factor) ratio ≤ 38, which rules out suspected preeclampsia,11 although it still has little clinical dissemination.
Several controversial aspects concerning the pharmacological prevention of preeclampsia are addressed:
- •
Aspirin (100-150mg/d) from weeks 12 to 36 or 37 is now systematically recommended in women with a high-risk criterion (previous HT, kidney or autoimmune disease, diabetes mellitus types 1 and 2) or at least 2 moderate-risk criteria (first pregnancy, multiple pregnancy, age > 40 years, previous pregnancy > 10 years earlier, body mass index > 35, family history of preeclampsia).12
- •
Calcium supplementation (1.5-2.0g/d) is recommended from the antenatal visit if the usual intake is low (<600mg/d). However, vitamins C and D not only do not prevent HT, but also increase the risk of low fetal weight and perinatal problems.
- •
There is no evidence that fertility treatments increase the risk.
For the nonpharmacological treatment of HT, the effectiveness of a low-sodium diet is still considered to be poor and the gestational weight gain in obese women should still be limited to 6.8kg. Beta-blockers (labetalol and metoprolol), alpha-methyldopa, and calcium antagonists (nifedipine) remain the drug treatments of choice for moderate HT. There are no changes in the management of severe HT, empirically defined by blood pressure values ≥ 170/110mmHg: intravenous labetalol, alpha-methyldopa, and oral calcium antagonists are still indicated, and intravenous urapidil can be used. In hypertensive crises associated with pulmonary edema, intravenous nitroglycerin is still recommended, as well as magnesium sulfate if there are seizures.
12VENOUS THROMBOEMBOLIC DISEASE DURING PREGNANCY AND THE PUERPERIUMVenous thromboembolism occurs in these periods in up to 0.2% of pregnancies. Pulmonary thromboembolism remains one of the main causes of morbidity and mortality. Its high recurrence is associated with several identifiable risk factors. Prevention involves the antenatal, gestational, and postpartum periods, and the patient at risk should be informed as a class I recommendation. There are no changes in these guidelines regarding the prevention or management of thromboembolic events.
For prevention, the drug of choice is LMWH in body weight-guided doses (enoxaparin 0.5 IU/kg/d or equivalent doses of dalteparin or fondaparinux), which allow a better concentration of adequate factor Xa inhibitors, particularly in obese women. The pharmacokinetics vary considerably during pregnancy and a dose increase of up to 50% may be required. Prophylactic treatment should continue in the puerperal period, for 6hours after a vaginal delivery and 12 after a cesarean delivery.
The technique of choice for the diagnosis of deep vein thrombosis in pregnant women is complete compression ultrasonography. Serial compression ultrasonography has a high negative predictive value in women with suspected deep vein thrombosis,13 although it is more effective for proximal thrombosis than for distal and pelvic thrombosis. The D-dimer is not specific and increases progressively during pregnancy. Because clinical diagnosis of pulmonary thromboembolism is difficult, particularly in this setting, the condition should be confirmed using any imaging tests deemed necessary. The treatment of choice for deep vein thrombosis or pulmonary thromboembolism is also LMWH at therapeutic doses (enoxaparin 1mg/kg/12h or equivalent).
For pregnant women on anticoagulant therapy, delivery should be planned for about week 39 to allow partial reversion with protamine sulfate or a switch to intravenous unfractionated heparin. If the bleeding risk is high, the addition of oxytocin in the third stage of labor is associated with less blood loss without hemodynamic deterioration.14
13DRUGS DURING PREGNANCY AND BREASTFEEDINGThe guidelines show a notable lack of uniform recommendations in this section. Before the initiation of any drug treatment during pregnancy, the guidelines recommend that physicians consult a table with data on the clinical safety of the drug or verify the regimen through well-established websites. If there is insufficient information on the safety of human administration, all decisions should be made by assessing the safety and efficacy profile of the drug, in consultation with the patient (IIa C). The guidelines do not recommend decisions based on FDA categories (III C).
Strikingly, the drugs most commonly used in cardiology are either completely contraindicated (statins, ACEIs/ARA-IIs, aldosterone inhibitors) or discouraged (atenolol) in pregnancy.
14CONCLUSIONSThe new guidelines emphasize the benefit of risk stratification of pregnant women with heart disease according to the mWHO classification. Women at moderate or high risk of complications during pregnancy (mWHO II-III, III, and IV) should receive prepregnancy counseling and management during pregnancy from a multidisciplinary pregnancy heart team. The approach during pregnancy should be individualized according to maternal risk and performed in expert centers when required. In addition, vaginal delivery is strongly favored when there are no formal cardiological contraindications. Medical therapy should always be assessed based on current preclinical safety data, and the use of the FDA classification is not recommended.
15CONFLICTS OF INTERESTNone declared.
SEC Working Group for the 2018 ESC Guidelines for the Management of Cardiovascular Diseases During Pregnancy: Manuel Jiménez Navarro (coordinator), Laura Galian-Gay (coordinator), Pablo Avanzas, Sara Ballesteros, Ana González García, Ernesto González Mesa, Isaac Martínez Bendayán, Antonia Pijuan Domenech, Raquel Prieto, and M. Teresa Subirana.
Expert Reviewers for the 2018 ESC Guidelines for the Management of Cardiovascular Diseases During Pregnancy: José Luis Bartha, Juan Caro, Juan Luis Delgado, Begoña Manso, Sandra Rosillo, and J. Ignacio Zabala.
SEC Guidelines Committee: Fernando Alfonso, Borja Ibáñez, Fernando Arribas, Gemma Berga Congost, Héctor Bueno, Arturo Evangelista, Ignacio Ferreira-González, Manuel Jiménez Navarro, Francisco Marín, Leopoldo Pérez de Isla, Antonia Sambola, Rafael Vázquez, and Ana Viana-Tejedor.