The aim of this editorial is to present the most relevant and innovative aspects of the new European Society of Cardiology (ESC) 2020 Guidelines for the Management of Adult Congenital Heart Disease (CHD),1 in line with the recommendations of the Clinical Practice Guidelines established by the Executive Committee of the Spanish Society of Cardiology.2
This new version of the guidelines is an update of the guidelines published in 20103 based on a systematic review of advances made over the past 10 years.
The guidelines use a new format indicating changes made to the previous guidelines3 (table 1), as well as an extremely useful series of algorithms to understand the recommendations, new sections listing situations with a lack of evidence, and summaries of key messages. The section on specific lesions now includes a segment on coronary anomalies, of considerable interest for cardiologists not specifically focused on CHD. Additionally, the term “grown-up congenital heart disease” has been replaced with “adult congenital heart disease”, which is more appropriate and in keeping with standard practice.
New and revised recommendations
| Arrhythmia | ||
| N | • Consideration of early catheter ablation as an alternative to long-term medical treatment for symptomatic SVT and VT provided that the procedure is performed in experienced centres• Targeting VT-related anatomical isthmuses in repaired tetralogy of Fallot patients with sustained VTs before percutaneous or surgical reintervention in RVOT, as these procedures may lead to inaccessibility of the VT substrates | |
| Eisenmenger syndrome/pulmonary artery hypertension | ||
| R | Emphasize the importance of sequential PAH therapy strategy in Eisenmenger syndrome and use of 6MWT for the decision to initiate therapy. | In Eisenmenger patients with reduced exercise capacity (6MWT distance < 450 m), a treatment strategy with initial endothelin receptor antagonist monotherapy should be considered followed by combination therapy if patients fail to improve. |
| Shunt lesions | ||
| N | In patients with shunt lesions and non-invasive signs of PAP elevation, invasive measurement of PVR is mandatory. | |
| N/R | Adjusted recommendations for shunt closure (when Qp:Qs > 1.5) according to calculated PVR: | |
| ≥ 5 WU but decreasing to <5 WU after targeted PAH treatment: class IIb for ASD (fenestrated closure only) | ||
| ≥ 5 WU for VSD and PDA (careful individual decision in expert centres); class IIb | ||
| N | In patients with ASD and LV disease, it is recommended to perform balloon testing and carefully weigh the benefit of eliminating L-R shunt against the potential negative impact of ASD closure on outcome due to increase in filling pressure (taking closure, fenestrated closure, and no closure into consideration). | |
| Left ventricular outflow tract obstruction and aortopathies | ||
| R | Lower mean Doppler gradient threshold for LVOTO intervention from 50 to 40mmHg. | In symptomatic patients with valvular, subvalvular, or supravalvular AS and mean Doppler gradient ≥ 40mmHg, surgery is recommended |
| N | Catheter treatment (stenting) of coarctation should be considered in normotensive patients with an increased non-invasive gradient confirmed with invasive measurement (peak-peak ≥ 20 mmHg) when technically feasible. | |
| Right ventricular outflow tract obstruction/tetralogy of Fallot/Ebstein | ||
| R | Include preference for catheter intervention for pulmonary valve implantation in TOF. | In patients with no native outflow tract, catheter intervention (TPVI) should be preferred if anatomically feasible. |
| R | Specify RV dilatation in the setting of pulmonary valve replacement for TOF and for RVOT conduits. | Pulmonary valve replacement should be considered in asymptomatic patients with severe PR and/or RVOTO, in the presence of progressive RV dilation to RVESVi ≥ 80 mL/m2, and/or RVEDVi ≥ 160 mL/m2, and/or progression of TR to at least moderate. |
| Transposition of the great arteries | ||
| N | In ccTGA, biventricular pacing should be considered in case of complete AV block or > 40% ventricular pacing requirement. | |
| Fontan circulation | ||
| N | Sustained atrial arrhythmia with rapid AV conduction is a medical emergency and should promptly be treated with electrical cardioversion > 40% | |
| N | Regular liver imaging (ultrasound, computed tomography, magnetic resonance) should be considered. | |
| N | Endothelin receptor antagonists and phosphodiesterase-5 inhibitors may be considered in selected patients with elevated pulmonary pressures/resistance in the absence of elevated ventricular end diastolic pressure. | |
| Coronary artery anomalies | ||
| N | Non-pharmacological functional imaging (e.g. nuclear study, echocardiography or CMR with physical stress) is recommended in patients with coronary anomalies to confirm/exclude myocardial ischaemia. | |
AS, aortic stenosis; ASD, atrial septal defect; AV, atrioventricular; ccTGA, congenitally corrected transposition of the great arteries; CMR, cardiovascular magnetic resonance; LVOTO, left ventricular outflow tract obstruction; 6MWT, 6-minute walk test; PAH, pulmonary artery hypertension; PAP, pulmonary artery pressure; PDA, patent ductus arteriosus; PR, pulmonary regurgitation; PVR, pulmonary vascular resistance; RVEDVi, right ventricular end diastolic volume indexed; RVOT, right ventricular outflow tract; RVOTO, right ventricular outflow tract obstruction; SVT, supraventricular tachycardia; TOF, tetralogy of Fallot; TPVI, transcatheter pulmonary valve implantation; TR, tricuspid regurgitation; VSD, ventricular septal defect; VT, ventricular tachycardia; WU, Wood units.
Summarized version of Table 3 by Baumgartner et al.1 Reproduced with permission from the European Society of Cardiology and Oxford University Press.
One of the main objectives of clinical practice guidelines is to support recommendations with as much as evidence as possible. This specialty covers a broad range of diseases, and the number of patients is often low, with only a few randomized studies or meta-analyses. Only 3 recommendations have a level of evidence B, and 1 has level A (all from previously published guidelines), most of them based on expert consensus and including several controversial recommendations, such as removal of the acute vasodilator test, inclusion of the treat-and-repair strategy or pulmonary vascular resistance (PVR) limits in decision-making for atrial septal defect (ASD) closure, pulmonary vasodilator therapy in Eisenmenger syndrome, and cutoff points for right ventricle (RV) volume for pulmonary valve replacement in tetralogy of Fallot. Moreover, the broad heterogeneity of the adult CHD patient population make individualization in decision-making inevitable. Nevertheless, these guidelines are a fundamental tool for assessing this population and add essential and highly practical information for management.
GENERAL ASPECTSOrganization of careThe ESC 2020 document emphasizes recommendations for the transition from pediatric to adult care and underscores the need to establish advanced care planning by creating multidisciplinary teams in specialized facilities.4,5 It outlines the human resources that should be offered by all specialized units, now featuring, in comparison with prior guidelines,6 the inclusion of specialist nurses, experts in pulmonary vasculature diseases, geneticists, and a palliative care team.
The previous guidelines3 stratified the care of patients into 3 levels: patients who need care exclusively in specialized facilities, patients whose care can be shared with general cardiology units, and patients who can be treated in nonspecialized units, with access to specialized care if necessary. The new guidelines maintain these recommendations, assert that the degree of complexity should not be the only criterion for assigning patients to a certain level of care, and advocate that all patients be assessed at least once by a specialized unit that will decide the necessary level of care and follow-up intervals. An updated table categorizes levels of complexity and reclassifies patients with transposition of the great arteries surgically repaired by the arterial switch procedure, now placing them in the moderate complexity category.
Diagnostic work-upA multimodal approach is necessary for complete patient assessment in a multidisciplinary setting, allowing CHD imaging experts to interact with other specialists (eg, surgeons, interventional cardiologists, electrophysiologists). Cardiopulmonary stress testing is considered key to objectively defining the patient's clinical status and optimal timing for surgery, mentioning that the test should be part of follow-up protocols and introducing for the first time the usefulness of biomarkers in patients’ clinical progress.
Pregnancy, contraception, and genetic counselingThis section is highly relevant and includes a table classifying conditions with a high or extremely high risk of pregnancy-related complications. The latter group includes patients with pulmonary hypertension or Fontan circulation with clinical complications, in whom formal contraindication of pregnancy is recommended.
HEART FAILUREAlthough specific diagnostic criteria are not established, the importance of actively screening high-risk patients is emphasized, as such patients often exhibit atypical symptoms.7
The section on biomarkers mentions that BNP and NT-ProBNP are less useful for diagnosing heart failure (HF) due to the lack of cutoff points for different cardiac lesions. Serial testing as a prognostic factor is recommended, although the utility of the biomarkers is limited in patients with Fontan surgery. Furthermore, hypoxia is mentioned as a possible inducer of peptide secretion, hence increased peptides in cyanotic patients should be interpreted within the clinical context.
Any significant structural lesion, including arrhythmias, should be treated surgically or percutaneously in all patients who experience a new HF episode. The pathophysiologic heterogeneity of HF in these patients limits the extrapolation of results from pharmacologic studies carried out in patients with acquired heart disease. Standard HF treatment is recommended for all patients with biventricular circulation and left ventricular dysfunction and, for the first time, also for symptomatic patients with systemic RV dysfunction; the guidelines include no recommendations on sacubitril/valsartan or sodium–glucose cotransporter type 2 inhibitors, as there is no evidence in this population. The utility of cardiac resynchronization therapy is mentioned, although its indications are still being researched.
It is recommended that acute HF be treated at experienced centers able to start advanced treatments, such as extracorporeal membrane oxygenation (ECMO) or ventricular assist. Additionally, the guidelines propose that heart transplant candidates be assessed at transplantation hospitals with cardiologists specialized in HF within CHD, one of the most innovative concepts in the current version.8 However, there is no mention that the multidisciplinary team evaluating these patients should also include other specialists with experience in CHD, such as cardiac surgeons, anesthesiologists, intensivists, and hepatologists.9
ARRHYTHMIASThe section on arrhythmias and sudden cardiac death (SCD) has been expanded considerably in this version and highlights the importance of understanding the potential mechanisms involved and the anatomy underlying arrhythmias in these patients. It also includes a table with clear, visual information on the risk of the various types of arrhythmia according to CHD, in particular the potential risk of hemodynamic decline and sudden death in supraventricular arrhythmias with rapid atrioventricular (AV) conduction.
Regarding diagnosis, there is a mention of the questionable role of Holter monitoring in screening for tachyarrhythmias in asymptomatic patients, given the high prevalence of events with no clinical or therapeutic implications, in contrast with its usefulness in screening for sinus node dysfunction and atrioventricular block.
Patients with CHD, particularly those with moderately or severely complex heart disease, should be assessed by multidisciplinary teams in facilities experienced in managing adults with CHD and associated arrhythmias (class I); the importance of maintaining sinus rhythm is emphasized, particularly in more complex heart diseases. In cases of sustained, symptomatic, and recurrent supraventricular tachyarrhythmia, catheter ablation is proposed as a first option over pharmacologic treatment whenever possible. Long-term oral anticoagulant therapy is recommended for atrial fibrillation (AF) or intra-atrial reentrant tachycardia (IART) in patients with moderate or complex CHD, whereas in patients with a simple CHD, the CHA2DS2-VASc score should be used for decision-making. For patients not receiving anticoagulation who have AF or IART> 48hours, the need for transesophageal echocardiography (TEE) before cardioversion is mentioned, but there are no recommendations for episodes <48hours, an issue addressed in the new version of the AF guidelines published at the same time.10 In these cases, the complexity of the heart disease and the patient's clinical status should be considered when evaluating the need for TEE before cardioversion.
The indication for pacemaker implantation is proposed for patients with bradycardia-tachycardia syndrome to prevent IART (class IIa) when ablation is ineffective and to resynchronize patients with congenitally corrected transposition of the great vessels (systemic RV) who have complete AV block or require pacing> 40%, and His bundle pacing is mentioned as an alternative to conventional resynchronization.
The section on SCD and risk stratification describes, along with known uncertainties,7 the most commonly accepted indications in CHD for an implantable cardioverter defibrillator (ICD) for either primary or secondary prevention. Subcutaneous ICD is listed as an alternative for patients with anatomic difficulties, provided that they do not require pacing and in view of the limitations of oversensing and lack of antitachycardia pacing.
PULMONARY HYPERTENSIONFor the first time, the new definition of precapillary pulmonary hypertension (PH) is included in clinical practice guidelines, reducing the cutoff point for mean pulmonary artery pressure from 25 to 20mmHg, while the cutoff points of PVRs≥ 3WU and pulmonary capillary pressure ≤ 15mmHg remain unchanged.
Risk assessment is introduced as a guide for treatment, and initial or sequential combination treatment is recommended.
In Eisenmenger syndrome, endothelin receptor antagonist therapy is now generally recommended for patients with reduced exercise capacity (defined as distance <450 m in the walk test), instead of according to functional class. In the absence of response, combined treatment is recommended. If necessary, inhaled or subcutaneous prostacyclin therapy is recommended instead of intravenous epoprostenol, in order to prevent complications associated with a permanent central intravenous catheter.
There are no long-term therapy recommendations for patients with elevated PH and PVRs that contraindicate shunt closure or for patients with segmental PH.
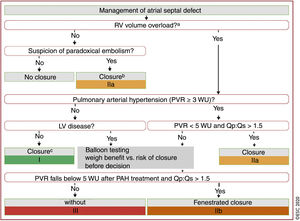
SPECIFIC LESIONSShuntsPretricuspid shuntsTemporary balloon occlusion of the defect is proposed to assess the behavior of the left ventricle (LV) end-diastolic pressure and to evaluate the indication for closure to treat ASD in patients with high LV pressures. Right-heart catheterization and exercise testing are recommended for patients with evidence consistent with PH (systolic pulmonary artery pressure> 40mmHg on echocardiography), in order to ascertain PVRs, the quotient between pulmonary and systemic flow (QP/QS), and the presence of exercise-induced systemic desaturation. These aspects are key for indicating shunt closure. PVRs <5 WU with QP/QS> 1.5 are established as the upper limit of PH in ASD to allow defect closure. Indications for closure (figure 1) are more restrictive than in the 2010 guidelines and definitively contraindicate closure in the case of PVRs > 5WU after pulmonary vasodilator therapy.
Management of atrial septal defect. Reproduced from Baumgartner et al.1 with permission from the European Society of Cardiology and Oxford University Press. ASD, atrial septal defect; L-R, left-to-right; LV, left ventricle/ventricular; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; Qp:Qs, pulmonary to systemic flow ratio; RV, right ventricle/ventricular; WU, Wood units. aRV enlargement with increased stroke volume. bProviding there is no PAH or LV disease. cIn elderly patients not suitable for device closure, carefully weigh surgical risk vs. potential benefit of ASD closure. dCarefully weigh the benefit of eliminating L-R shunt against the potential negative impact of ASD closure on outcome due to an increase in filling pressure (taking closure, fenestrated closure, and no closure into consideration).
The treat-and-repair strategy is recommended over acute vasodilator testing to assess shunt closure, although the level of recommendation is only IIb. Because patients with precapillary PH receiving dual therapy initially achieve PVR reductions of 50% only for a short time,11 interpreting PVR reduction in response to specific PH treatment as assurance that the ASD is closed is considered unclear, particularly when also considering that PH may appear after ASD repair years after closure and has a poor prognosis.12
Post-tricuspid shuntsIn posttricuspid shunts, PH is assessed differently and allows higher PH levels for closure. Closure is allowed with PVRs ≥ 5WU, provided that QP/QS> 1.5 is maintained and assuming that this circumstance will be extraordinarily rare in adults and will be assessed at a facility with experience in managing these patients. In these cases, there is no indication regarding the validity of treat-and-repair or the usefulness of acute vasodilator tests to assess PH reversibility.
The algorithm used to indicate shunt closure begins with LV volume overload, but still does not specify cutoff points or specify a quantification method. This point is particularly controversial in patients with severe PH (PVR> 5WU), in whom the appearance of LV volume overload is practically impossible because of the logical reduction in left-to-right shunt due to increased pressures. Furthermore, it can lead to inaccurate interpretations because PVR limits that contraindicate closure are not established. Additionally, the guidelines do not include the PVR to systemic vascular resistance quotient, which is a critical point when assessing shunt closure in pediatric patients.
Ductus closure is now indicated only on the basis of hemodynamic criteria, and the indication due to auscultation of a continuous murmur has been removed.
LEFT-SIDED OBSTRUCTIVE HEART LESIONS AND AORTOPATHIESUpdates to the guidelines can be summarized as the need to individualize the recommendations according to the type of aortopathy or obstruction location, the lower thresholds for indicating surgery, the importance of invasive diagnosis, and the preference for percutaneous techniques and valve repair as the first option. In addition, heritable aortic diseases other than Marfan syndrome are included for the first time.
Valvular, subvalvular, and supravalvular aortic stenosisThe new indications for aortic stenosis are consistent with the 2017 guidelines on valve disease,13 the mean gradient for indicating surgery has been lowered to 40mmHg, and a class I indication has been established for low-flow/low-gradient stenosis in symptomatic patients with ventricular dysfunction and evidence of contractile reserve. Indicators of poor prognosis, such as PH or biomarker elevation, have also been included as a classIIa indication in asymptomatic patients at low surgical risk. Echocardiographic screening is now recommended for first-degree relatives of patients with bicuspid valve (BAV), particularly in people at greater risk for aortic complications (boys, athletes, and if hypertension is present).
In general, the threshold for indicating surgery in asymptomatic patients at low surgical risk in subvalvular and supravalvular aortic stenosis is lower than in valvular stenosis, particularly when it is possible to spare the valve and thus avoid exposing young patients to the cumulative risk of valve replacement-related complications. Potentially of greater interest are updates on the diagnostic approach to both lesions: a recommendation is included on assessment and genetic counseling using microarray techniques if Williams-Beuren syndrome is suspected, and elastin gene sequencing in nonsyndromic presentations of aortic supravalvular stenosis; multimodal imaging is suggested for some forms of subvalvular stenosis and, more importantly, the usefulness of invasive diagnosis is emphasized, particularly for more complex anatomic lesions. Unlike acquired lesions, which are simple and localized, congenital lesions are generally multiple, of tubular morphology, with eccentric jets, and often associated with significant aortic regurgitation or shunts. Because of these features, the modified Bernoulli equation (with velocity near stenosis) should be used to calculate the gradient, and several echocardiographic planes should be used to determine accurate maximal velocities, which means the Doppler-derived gradient accuracy is limited.14
Aortic coarctation and heritable thoracic aortic diseaseIn aortic coarctation, it is recommended that the gradient be confirmed by an invasive study, with a preferential indication of percutaneous treatment over surgery, with even less stringent criteria for stent implantation in normotensive patients with invasive peak-to-peak gradients ≥ 20mmHg (class IIa). The indications of medical and surgical treatment are individualized according to the type of aortopathy, emphasis is given to genetic diagnosis and to adjusting the aorta dimensions to body surface area in patients with Turner syndrome, and the risk of bicuspid aortopathy is minimized with respect to other syndromic or familial forms. Nevertheless, the guidelines recommend that pregnancy be contraindicated in women with BAV and aorta> 50mm. Last, they also stress the relatively high risk of recurrence in offspring, in the case of both left-sided obstructive heart lesions and aortopathies.
RIGHT-SIDED OBSTRUCTIVE HEART LESIONS AND TETRALOGY OF FALLOTRight ventricular outflow tract obstructionIn general, the indications for surgery are stricter if pulmonary valve replacement is required, and a useful flow chart is included to assist in decision-making. Percutaneous balloon valvuloplasty is the treatment of choice in cases of nondysplastic pulmonary stenosis, without hypoplastic annulus and with no need for surgery for another cause. It is worth remembering that pulmonary artery aneurysms, at low risk of rupture, do not usually require surgery.
Tetralogy of FallotIn patients with asymptomatic severe pulmonary regurgitation, cutoffs are established for progressive RV as an indication of valve replacement. Normalization of RV volumes after pulmonary valve replacement is unlikely if they exceed 80mL/m2 for end-systolic volume or 160mL/m2 for end-diastolic volume, although clinical benefit has not yet been shown with this improvement in RV remodeling.
One of the most noteworthy innovations is that percutaneous pulmonary valve implantation has become the treatment of choice, if technically feasible, for patients who already have a bioprosthesis or impaired ducts, currently with a class I15 recommendation. The reason for this indication (with a more robust recommendation level than for aortic valves in the guidelines for valve disease) is the attempt to minimize the number of reinterventions among the target population, usually with 1 or more prior surgeries. Accordingly, the risk of infective endocarditis should be taken into account when considering the expansion of the indications for percutaneous pulmonary valve replacement.
In the case of a class IIa recommendation (IIb in the previous guidelines), an electrophysiological study (programmed electric pacing) is recommended in patients meeting the risk criteria for SCD, in order to establish the indication for ICD implantation for primary prevention. However, the number of risk criteria to be taken into account (for performance of the study and for the ICD indication) is not specified, nor is ablation indicated for these inducible ventricular tachycardias (VT). Nevertheless, when patients with clinical VT require surgical or percutaneous pulmonary valve implantation, it is recommended (class IIa) that they undergo a preoperative electrophysiological study and ablation of isthmuses that could be less accessible after implantation. The benefit of systematic ablation of slow conduction isthmuses before pulmonary valve replacement is still being researched.
TRANSPOSITION OF THE GREAT ARTERIESAtrial switchAlthough there are no new indications, the class of recommendation has been lowered for the repair or replacement of systemic tricuspid valve with severe regurgitation in symptomatic patients, specifying that the intervention should be performed without relevant ventricular dysfunction (RVEF ≥ 40%), regardless of symptoms (class IIa).
More arrhythmic events, particularly supraventricular, appear with age and affect both quality of life and survival. Catheter ablation is recommended for isthmus-dependent flutter by transbaffle puncture, and programmed electric pacing is mentioned as not being useful for risk stratification, although no evidence has been published to support this statement.16
Arterial switchThe usefulness of magnetic resonance imaging (MRI) is highlighted as useful to assess the anatomy of the pulmonary trunk and its branches and to analyze perfusion distribution, without requiring additional nuclear techniques. Reduced pulmonary perfusion is one of the indications for percutaneous treatment of pulmonary branch stenosis, and stenting is confirmed as preferable over surgery.
Potential coronary involvement is deemed relatively unimportant, due to its scant prevalence among adults, and routine screening with imaging techniques is even questioned, emphasizing that surgery on the neoaortic root should be considered when the diameter is> 55mm.
Congenitally corrected transposition of the great arteriesThe indication for double switch in adulthood is definitively removed due to its high surgical risk. The level of recommendation (class I) for tricuspid valve replacement is increased for symptomatic patients provided systemic RVEF> 40%. Conversely, asymptomatic patients must also have progressive dilation or mild RV dysfunction, and systemic RVEF should be > 40% (class IIa).17
There is no recommendation for pharmacologic treatment of asymptomatic patients to improve the prognosis. Additionally, the viability of device-based ventricular assist is discussed, and RV trabeculectomy may be required for proper cannula function.
UNIVENTRICULAR HEART AND FONTAN CIRCULATIONUniventricular heartPalliative interventions in these patients should always be restricted to highly selected cases and after a careful assessment. The updated guidelines include the recommendation (although class IIb) to create a systemic-pulmonary shunt as potential treatment for patients with severe cyanosis and diminished pulmonary flow not eligible for a Glenn-type connection.
FontanImaging tests are emphasized, whether MRI or computed tomography (CT) for anatomic and functional assessment of collaterals, thrombi, and flow through the cavopulmonary connection, and the importance of the hepatic study is discussed in terms of liver function and screening for advanced stages of cirrhosis and hepatocellular carcinoma. Periodic study is recommended with imaging tests, sonography, and MRI/CT on the liver plus lab work, including alpha-fetoprotein testing, with a baseline assessment and regular follow-up based on each patient's situation.
The possibility of pulmonary vasodilator treatment is considered for patients with elevated pulmonary/resistance, provided that single-ventricle end-diastolic pressure is not elevated (class IIb), although its usefulness has only been shown in cases with decreased functional capacity.
Because loss of sinus rhythm and atrial arrhythmias can lead to severe hemodynamic decline, the role of early electrical cardioversion is enhanced, and catheter ablation is proposed as the first therapeutic option.
In the new version, the threshold has been lowered for cardiac catheterization in the case of clinical complications, but the procedure is not considered a routine diagnostic test for stable patients to allow a more accurate anatomic and hemodynamic assessment than with other techniques.18
The indication of anticoagulant therapy is still a controversial issue, although the same recommendations are established: atrial thrombi, arrhythmias, or thromboembolic events. Direct-acting anticoagulants are a possible option, with safety and efficacy based only on registry data, with no randomized studies to support them.
Protein-losing enteropathy may be treated by transplantation when medical treatment fails, but no criteria are established for heart or liver transplant in Fontan.19
CORONARY ANOMALIESAnomalous aortic origin of a coronary arteryAnomalous aortic origin of the right coronary artery (AAORCA) from the left aortic sinus is 6- to 10-fold more common than anomalous origin of the left coronary artery (AAOLCA) from the right aortic sinus, but the higher risk is associated with the latter as a cause of SCD in individuals younger than age 35years who practice strenuous physical exercise. Diagnosis requires assessing anatomic and clinical aspects and proving inducible ischemia with imaging techniques based on nonpharmacologic stress, although the sensitivity of these techniques is low, particularly in AAORCA. CT is the technique of choice, as it provides more information than invasive coronary angiography.
Surgical indications are controversial for several reasons: the prognostic value of the various anatomic and clinical risk factors, the high variability of the surgical techniques described, with no evidence on the prevention of SCD for each one, and the persistence of ischemia or symptoms in some patients after surgery.20 These considerations must be taken into consideration, along with surgical experience to interpret the indications of the guidelines. Surgery is considered reasonable for patients with AAORCA or AAOLCA and demonstrable ischemia regardless of clinical symptoms and for patients with asymptomatic AAOLCA and no ischemia, but with risk anatomy (classIIa). It is not considered indicated for asymptomatic patients with AAORCA without ischemia or risk anatomy (class III), and there is no evidence for drug therapy, exercise restrictions, or arrhythmia studies.
Anomalous coronary artery from the pulmonary arteryPatients with an abnormal coronary artery from the pulmonary artery have elevated mortality in childhood, but can survive to adulthood by developing intercoronary collateral circulation. The clinical course varies from silent forms to HF, arrhythmias, etc. The anomalous origin may be from either coronary artery: left (ALCAPA) or right (ARCAPA), which has fewer repercussions. ALCAPA cases are always surgical, whereas in ARCAPA, surgery is reserved for symptomatic cases with ventricular dysfunction or demonstrable ischemia.
CONCLUSIONSThe cardiologic care of adults with CHD should focus on multidisciplinary care in specialized units that can offer clinical experience and all the diagnostic and therapeutic techniques required by this complex, heterogeneous population. These guidelines lay down recommendations based on currently available evidence, but there are still considerable gaps in the knowledge of these diseases that we hope to clarify in upcoming years through multicenter network research and the use of new methodologies (artificial intelligence).
CONFLICTS OF INTERESTThe authors declare no conflicts related to this article.
SEC Working Group for the ESC 2020 Guidelines for the management of adult congenital heart disease: Laura Dos (coordinator), Joaquín Rueda Soriano (coordinator), Pablo Ávila, Pilar Escribano, M. Elvira Garrido-Lestache Rodríguez-Monte, Ana Elvira González, Isaac Martínez Bendayan, Sílvia Montserrat, Pastora Gallego, Rafael Alonso, and M. Antonia Martínez Momblán.
Expert reviewers for the ESC 2020 for the management of adult congenital heart disease: Rocío García Orta, José María Oliver Ruiz, Rafael Peinado Peinado, Óscar Cano Pérez, Federico Gutiérrez Larraya, Ariana González, Irene Méndez, and María Lázaro Salvador.
SEC Guidelines Committee: Pablo Avanzas, Gemma Berga Congost, Araceli Boraita, Héctor Bueno, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos, Ignacio Ferreira-González, Juan José Gómez Doblas, Domingo Pascual Figal, Antonia Sambola Ayala, Ana Viana Tejedor, José Luis Ferreiro (copresident), and Fernando Alfonso (copresident).
.