Cardio-oncology is a growing field that covers the diagnosis and management of cardiovascular disease (CVD) in patients with cancer and focusing especially on cancer therapy-related cardiovascular toxicity (CTR-CVT). The European Society of Cardiology (ESC) recently presented the first cardio-oncology guidelines.1 All the information contained in these new guidelines is long awaited and relevant: it is useful and excellently presented and will have an important impact on the management of oncohematologic patients treated with potentially cardiotoxic antitumoral therapies.
In line with all the ESC guidelines, the document was produced in collaboration with other international societies related to the topic and followed a strict protocol for the literature review, recommendations, and supporting evidence. A serious review of each topic was carried out, although, because of the scarcity of published evidence in cardio-oncology, 76% of the recommendations have a level of evidence C, highlighting the need for continuous research. Of note, the document is long, with 133 pages and 272 new recommendations that imply multidisciplinary collaboration and resources, which may be difficult to apply, especially in centers that are starting their clinical activity in cardio-oncology. Therefore, it is important to carefully review the guidelines and identify the recommendations that can realistically be implemented in Spain and which will provide the greatest benefit to our patients with cancer.
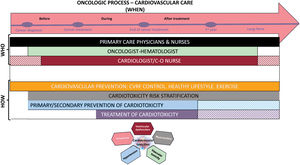
This commentary aims to provide a detailed discussion of each section of the 2022 ESC cardio-oncology guidelines and delve into potential strategies for implementing the recommendations in our health system. The main positive and controversial aspects and local considerations are summarized in table 1, while figure 1 represents the global approach throughout the continuum of cardiovascular care in these patients.
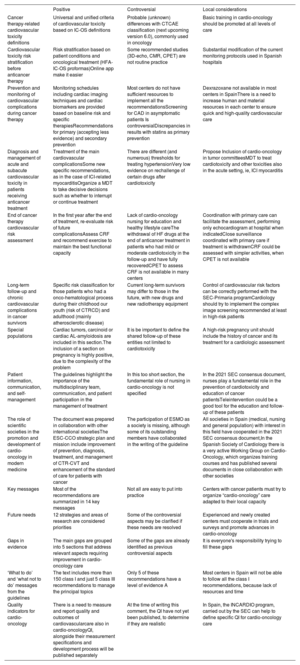
Main positive and controversial aspects and local considerations
| Positive | Controversial | Local considerations | |
|---|---|---|---|
| Cancer therapy-related cardiovascular toxicity definitions | Universal and unified criteria of cardiovascular toxicity based on IC-OS definitions | Probable (unknown) differences with CTCAE classification (next upcoming version 6.0), commonly used in oncology | Basic training in cardio-oncology should be promoted at all levels of care |
| Cardiovascular toxicity risk stratification before anticancer therapy | Risk stratification based on patient conditions and oncological treatment (HFA-IC-OS proformas)Online app make it easier | Some recommended studies (3D-echo, CMR, CPET) are not routine practice | Substantial modification of the current monitoring protocols used in Spanish hospitals |
| Prevention and monitoring of cardiovascular complications during cancer therapy | Monitoring schedules including cardiac imaging techniques and cardiac biomarkers are provided based on baseline risk and specific therapiesRecommendations for primary (accepting less evidence) and secondary prevention | Most centers do not have sufficient resources to implement all the recommendationsScreening for CAD in asymptomatic patients Is controversialDiscrepancies in results with statins as primary prevention | Dexrazoxane not available in most centers in SpainThere is a need to increase human and material resources in each center to ensure quick and high-quality cardiovascular care |
| Diagnosis and management of acute and subacute cardiovascular toxicity in patients receiving anticancer treatment | Treatment of the main cardiovascular complicationsSome new specific recommendations, as in the case of ICI-related myocarditisOrganize a MDT to take decisive decisions such as whether to interrupt or continue treatment | There are different (and numerous) thresholds for treating hypertensionVery low evidence on rechallenge of certain drugs after cardiotoxicity | Propose Inclusion of cardio-oncology in tumor committeesMDT to treat cardiotoxicity and other toxicities also in the acute setting, ie, ICI myocarditis |
| End of cancer therapy cardiovascular risk assessment | In the first year after the end of treatment, re-evaluate risk of future complicationsAssess CRF and recommend exercise to maintain the best functional capacity | Lack of cardio-oncology nursing for education and healthy lifestyle careThe withdrawal of HF drugs at the end of anticancer treatment in patients who had mild or moderate cardiotoxicity in the follow-up and have fully recoveredCPET to assess CRF is not available in many centers | Coordination with primary care can facilitate the assessment, performing only echocardiogram at hospital when indicatedClose surveillance coordinated with primary care if treatment is withdrawnCRF could be assessed with simpler activities, when CPET is not available |
| Long-term follow-up and chronic cardiovascular complications in cancer survivors | Specific risk classification for those patients who had a onco-hematological process during their childhood our youth (risk of CTRCD) and adulthood (mainly atherosclerotic disease) | Current long-term survivors may differ to those in the future, with new drugs and new radiotherapy equipment | Control of cardiovascular risk factors can be correctly performed with the SEC-Primaria programCardiology should try to implement the complex image screening recommended at least in high-risk patients |
| Special populations | Cardiac tumors, carcinoid or cardiac AL-amyloidosis are included in this section.The inclusion of a section on pregnancy is highly positive, due to the complexity of the problem | It is be important to define the shared follow-up of these entities not limited to cardiotoxicity | A high-risk pregnancy unit should include the history of cancer and its treatment for a cardiologic assessment |
| Patient information, communication, and self-management | The guidelines highlight the importance of the multidisciplinary team, communication, and patient participation in the management of treatment | In this too short section, the fundamental role of nursing in cardio-oncology is not specified | In the 2021 SEC consensus document, nurses play a fundamental role in the prevention of cardiotoxicity and education of cancer patientsTeleintervention could be a good tool for the education and follow-up of these patients |
| The role of scientific societies in the promotion and development of cardio-oncology in modern medicine | The document was prepared in collaboration with other international societiesThe ESC-CCO strategic plan and mission include improvement of prevention, diagnosis, treatment, and management of CTR-CVT and enhancement of the standard of care for patients with cancer | The participation of ESMO as a society is missing, although some of its outstanding members have collaborated in the writing of the guideline | All societies in Spain (medical, nursing and general population) with interest in this field have cooperated in the 2021 SEC consensus document,In the Spanish Society of Cardiology there is a very active Working Group on Cardio-Oncology, which organizes training courses and has published several documents in close collaboration with other societies |
| Key messages | Most of the recommendations are summarized in 14 key messages | Not all are easy to put into practice | Centers with cancer patients must try to organize “cardio-oncology” care adapted to their local capacity |
| Future needs | 12 strategies and areas of research are considered priorities | Some of the controversial aspects may be clarified if these needs are resolved | Experienced and newly created centers must cooperate in trials and surveys and promote advances in cardio-oncology |
| Gaps in evidence | The main gaps are grouped into 5 sections that address relevant aspects requiring improvement in cardio-oncology care | Some of the gaps are already identified as previous controversial aspects | It is everyone's responsibility trying to fill these gaps |
| ‘What to do’ and ‘what not to do’ messages from the guidelines | The text includes more than 150 class I and just 5 class III recommendations to manage the principal topics | Only 5 of these recommendations have a level of evidence A | Most centers in Spain will not be able to follow all the class I recommendations, because lack of resources and time |
| Quality indicators for cardio-oncology | There is a need to measure and report quality and outcomes of cardiovascularcare also in cardio-oncologyQI, alongside their measurement specifications and development process will be published separately | At the time of writing this comment, the QI have not yet been published, to determine if they are realistic | In Spain, the INCARDIO program, carried out by the SEC can help to define specific QI for cardio-oncology care |
CAD, coronary artery disease; CMR, cardiac magnetic resonance; CPET, cardiopulmonary exercise stress test; CRF, cardiorespiratory fitness; CTCAE, common terminology criteria for adverse event; CTRCD, cancer therapy-related cardiac dysfunction; CTR-CVT, cancer therapy-related cardiovascular toxicity; ESC-CCO, European Society of Cardiology-Council of Cardio-Oncoloy; 3D, 3-dimensional; ESMO, European Society of Medical Oncology; HFA, ESC Heart Failure Association; ICI, immune checkpoint inhibitors; IC-OS, International Cardio-Oncology Society; MDT, multidisciplinary team; QI, quality indicators; SEC, Sociedad Española de Cardiología.
Cardiovascular complications secondary to cancer treatments may have a different clinical presentation than CVD in the general population and their diagnosis is crucial to minimize interruptions of oncological treatments. For this reason, it is highly recommended to standardize the definitions of the different adverse cardiovascular events. These include ventricular dysfunction, vascular toxicity, myocarditis, high blood pressure or long QT, among others. Universal diagnostic criteria for CTR-CVT will have important implications in the diagnosis, treatment, and follow-up of these patients, as well as for future research and clinical trials, which are essential in this population. The new ESC cardio-oncology guidelines confirm the new classification of cardiotoxicity published in 2022 as a consensus statement of the International Society of Cardio-Oncology (IC-OS).2
In our clinical practice, these criteria should be applied for clinical diagnosis, and we also should use these definitions in our medical reports. The cardio-oncology guidelines encourage the use of this classification and make it available to other specialists who also treat cancer patients.
Risk stratification before anticancer therapyThe cardio-oncology guidelines divide cardiovascular care for patients with cancer according to timing: before, during, and after cancer treatment. In a first stage, the main factors to be considered by oncologists and hematologists before treatment are cardiovascular risk stratification and prevention of cardiotoxicity.
It is recommended to evaluate cardiovascular and cardiotoxicity risk before treatment in all patients with cancer using the HFA-ICOS baseline risk assessment proformas.3 These scores are adapted for each type of cancer treatment and include information on previous CVD, imaging techniques, biomarkers, age and previous cardiovascular risk factors (CVRF) to classify cardiotoxicity risk as low, moderate, high, or very high. The text reminds us of the limitations of previous risk stratification scales. The HFA-ICOS proformas published in 2020 were created by expert consensus and were not previously validated. However, during the last ESC congress, a study confirmed the ability of this tool to successfully predict anthracycline (AC) cardiotoxicity in the CARDIOTOX registry cohort.4
The text highlights the role of basic elements such as clinical history and electrocardiography, which are highly valuable in the initial assessment. Regarding biomarkers, it is recommended to perform a baseline determination of troponin and/or natriuretic peptides (NP) if they are to be determined during follow-up to detect cancer therapy-related cardiac dysfunction (CTRCD). The preferred cardiac imaging method is transthoracic echocardiography (TTE): left ventricular ejection fraction (LVEF) and global longitudinal strain are recommended in all high- or very high-risk patients to assess cardiac function. Three-dimensional -LVEF measurement would be the ideal modality, although its availability is limited. If there is a poor acoustic window, cardiac magnetic resonance (CMR) should be considered for LVEF evaluation and multigated acquisition nuclear imaging when CMR is not possible. Genetic testing is not routinely indicated but the field is open to future personalized assessment.
A novel aspect is the indication of cardiorespiratory fitness assessment by a cardiopulmonary exercise stress test (CPET), which is a good predictor of cardiovascular health. Nevertheless, evidence for CPET pretreatment is limited to preoperative risk assessment in patients with cancer of the lung, colon, or rectum. Surgical risk assessment before cancer surgery should be carried out based on the type of tumor, risk factors, concomitant therapies, and surgical risk itself. Cardio-oncology assessment is recommended in symptomatic patients, with previous CVD, and high or very high risk of cardiotoxicity.
In summary, cardiovascular monitoring depends on both the antineoplastic treatment and the baseline risk of cardiotoxicity. This risk should be estimated by the oncologist or hematologist. To achieve this goal, training in cardio-oncology should be intensified, not only among cardiologists, but also among hematologists, oncologists, and radiation oncologists. As far as the applicability of pretreatment assessment in daily clinical practice in Spain is concerned, the app developed on the cardio-oncology pocket guides provides very useful and interactive tools for a rapid risk assessment. Until a few years ago, the only recommendation was evaluation with TTE prior to treatments such as AC or HER2-targeted therapies. The new guidelines now recommend TTE before other pharmacological groups such as proteasome inhibitors or osimertinib, even in low-risk patients (class I recommendation), which could currently be difficult to achieve considering the high workload in cardiac imaging units.
Prevention and monitoring of cardiovascular complicationsThe ESC cardio-oncology guidelines highlight that CTR-CVT risk is increased in patients with CVRF and previous CVD. General recommendations are given about healthy lifestyle, exercise, and CVRF correction. To facilitate the evaluation ofcardiovascularrisk and the optimization of CVRF in the Spanish health system, a previous consensus document elaborated by several Spanish scientific societies specifies the different evaluation scales and therapeutic objectives in these patients.5
For primary prevention, the guidelines recommend minimizing the use of potential cardiotoxic drugs if possible. Prescription of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ARB) and beta-blockers is recommended in high-risk or very high-risk patients (IIa B recommendation for AC or anti-HER2 treatments; IIa C for other treatments that may cause heart failure [HF]). Statins are also suggested for patients with high or very high cardiovascular risk at the baseline assessment. Other primary prevention strategies include the use of dexrazoxane before AC and the use of liposomal AC in adults with cancer at high risk of cardiovascular toxicity. The optimization of cardioprotective treatment or the use of liposomal AC are easily accessible strategies in Spain, but the indication of dexrazoxane is limited to advanced breast cancer, which will require a high cumulative dose of AC, and its availability is scarce in most hospitals.
For secondary prevention (patients with previous CVD, prior or new CVR-CVT), a multidisciplinary team (MDT) approach is encouraged. Cardiovascular assessment during anticancer treatment is recommended according to the baseline risk. In addition to the CTRCD generally associated with AC and HER2-targeted therapies, detailed recommendations are given on the detection and monitoring of coronary artery disease (eg, fluoropyrimidines or vascular endothelial growth factor inhibitors), hypertension (eg, vascular endothelial growth factor inhibitors or tyrosine kinase inhibitors), QTc prolongation (eg, vascular endothelial growth factor inhibitors and tyrosine kinase inhibitors such as nilotinib and dasatinib), pulmonary hypertension (eg, dasatinib), and screening for atrial fibrillation (AF) associated with ibrutinib (Bruton tyrosine kinase inhibitor), among others. These recommendations are graphically summarized in excellently designed figures. As previously mentioned, most of the recommendations are based on expert opinion (level of evidence C), which the authors acknowledge by stating that there is a significant lack of clinical trials to guide decision-making. Thus, of all the recommendations on cardiovascular monitoring, only 2 class I recommendations have level of evidence A: the use of low molecular weight heparin (LMWH) in the prophylaxis of venous thromboembolism (VTE) in patients with multiple myeloma and risk factors for VTE (excluding previous VTE) and the monitoring of QTc in patients treated with ribociclib.
Concerning radiotherapy, the use of the mean cardiac dose instead of the prescribed dose is recommended to accurately categorize the risk of cardiovascular toxicity which, additionally, may be modified according to the cardiac substructures irradiated and the dose distribution. This requires a substantial change in the way the radiation dose is reflected in the clinical reports in our hospitals.
The application of these guidelines requires a substantial modification of most of the current monitoring protocols, as well as the design of new protocols to include all the antineoplastic therapies discussed in the document. This implies a substantial increase in the number of cardiac biomarker determinations, electrocardiograms and TTE and, therefore, a greater number of specific cardio-oncology consultations. New structural and human resources will have to be planned in each center to ensure rapid and high-quality cardiovascular care, identify potential cardiovascular complications as early as possible, and avoid unnecessary interruptions of antineoplastic treatments. Until these increased resources are available, each center should evaluate the workload it can accept and select those patients who will derive the greatest benefit from this assessment, that is, those with the highest risk of cardiotoxicity. The guidelines do not specify their agreement with basic recommendations for those cardio-oncology units that are smaller or have fewer resources and we assume that individualized protocols should be developed locally.
Management of cardiovascular toxicityVentricular dysfunction and heart failureThe current guidelines provide specific recommendations on CTRCD due to AC, HER2-targeted therapies, chimeric antigen receptor T cell, tumor-infiltrating lymphocytes, HF during hematopoietic stem cell transplantation, immune checkpoint inhibitor myocarditis, and cancer-related tako-tsubo syndrome.
The management of AC CTRCD depends on whether patients have symptomatic HF or not and on the severity of ventricular dysfunction. Generally, patients with severe symptomatic cardiotoxicity will discontinue AC permanently; patients with moderate symptomatic cardiotoxicity and those with asymptomatic cardiotoxicity with LVEF<50% will temporarily discontinue AC and the MDT should discuss whether to rechallenge or not based on a risk-benefit assessment. In patients with mild symptomatic cardiotoxicity, it will also be the MDT who will decide whether to maintain or interrupt treatment with AC. The cardio-oncology guidelines aim to address those situations that differ in cancer patients, and medical treatment of ventricular dysfunction and symptomatic HF must follow ESC HF guidelines.6,7 In addition, 3 cardioprotective strategies are proposed for patients with CTRCD who restart AC, over HF treatment, and after LVEF recovery: dose reduction, liposomal AC, and dexrazoxane.
Mild asymptomatic CTRCD is a frequent clinical situation in cardio-oncology, defined as new global longitudinal strain decline>15% and/or an increase in cardiac biomarkers (either troponin or NP) with normal LVEF and without signs or symptoms of HF. Although the clinical evidence in this scenario is based on small studies and the results are heterogeneous, the guidelines recommend the initiation of angiotensin-converting enzyme inhibitors or beta-blockers in these patients to avoid progression of cardiotoxicity (IIa class B for those with troponin elevation or global longitudinal strain impairment; IIb class C for those with NP elevation).
Similar recommendations are given for CTRCD involving HER2-targeted therapies, but due to its less severe toxicity and its frequently reversible effect, the threshold for discontinuation is higher. This is an important message, because the fear of CTRCD leads to unnecessary interruption of anti-HER2 with potential oncologic benefit even though moderate cardiotoxicity can be tolerated.8 We believe that to apply all these recommendations, the work of cardio-oncology units should be integrated with that of HF units.
Immune checkpoint inhibitor-related myocarditis is clearly addressed. The 3 pillars of management are the following: a) to stop immune checkpoint inhibitors when suspected; b) to confirm diagnosis quickly: raised troponin, electrocardiogram, cardiac imaging (with a leading role of CMR), exclusion of other causes of myocardial injury such as acute coronary syndrome (ACS) or viral myocarditis; and c) to start high-dose boluses of methylprednisolone in all suspected patients, especially in fulminant cases where emergent differential diagnosis (including endomyocardial biopsy, IIaB) cannot be delayed. Despite the lack of an evidence-based recommendation, it is worth having a clear guide on how to manage corticoid weaning, when to use a second-line immunosuppressor and when to rechallenge (always through MDT) in this rare complication.
Readers are made aware of other specific HF complications in patients with cytokine releasing syndrome in chimeric antigen receptor T cell and tumor-infiltrating lymphocyte therapies, and in those receiving hematopoietic stem cell transplants, in whom cardiovascular toxicities include arrhythmias, pericardial effusion and tamponade, or HF from mild congestion to cardiogenic shock. Finally, tako-tsubo syndrome management in patients with cancer is approached and essential messages are given to confirm diagnosis, excluding ACS and myocarditis (again with an important role of CMR). The document stresses the avoidance of QT-prolonging drugs during the acute phase and the risk of restarting a cancer drug suspected of being the culprit drug after tako-tsubo syndrome should be preceded by MDT discussion.
Coronary artery diseasePatients with cancer are at an increased risk of coronary artery disease because of shared risk factors but also because of the prothrombotic state and the toxicity of many cancer therapies. Patients admitted because of an ACS have higher mortality and bleeding rates. Diagnosis of ACS should be the same as in patients without cancer. Percutaneous revascularization is safe and leads to better outcomes and is therefore recommended when life expectancy is ≥ 6 months (IB). Antithrombotic therapy should probably be less aggressive in most cancers due to the increased bleeding risk. The duration of dual antiplatelet therapy should be individualized, and short duration of clopidogrel strategy after PCI for ACS could be an option for patients with cancer and very high bleeding risk (IIa C).
Cancer therapies should be temporally interrupted when cancer therapy is suspected as a contributing cause. A rechallenge with fluoropyrimidines after a vasospasm is controversial but could be attempted, especially after prophylactic therapy with long-term nitrates and calcium channel blockers. Therapies not associated with ischemia can be restarted once the patient is stabilized. Patients with new stable angina during treatment should undergo careful clinical evaluation, aggressive risk factor modification, and initiate anti-ischemic drugs.
Valvular heart diseaseValvular heart disease increases the risk of cardiac toxicity. New or worsening valvular heart disease in patients with cancer may be related to coexisting conditions. Cardiac surgery is challenging because of comorbidities, frailty, or mediastinal fibrosis due to prior radiotherapy, and therefore percutaneous valve interventions may be an option to limit delays in starting cancer treatments. Patients with cancer suspected of new or worsening valvular heart disease should be screened for endocarditis and managed according to guideline recommendations but prioritizing the cancer-related prognosis.
Cardiac arrhythmiasAll types of cancer carry an increased risk of AF and are associated with a 2-fold higher risk of systemic thromboembolism/stroke and a 6-fold increase in the risk of heart failure. The management of AF in patients with cancer should initially follow the ‘ABC pathway’ of the 2020 ESC guidelines but could differ in these patients due to the clinical particularities and concomitant treatment. The Spanish cardio-oncology group has previously published a consensus document on this topic to guide management in our setting.9
Initiation of anticoagulation in new-onset cases of AF should be based on CHA2DS2-VASC and HAS-BLED scores and is recommended in adult patients with CHA2DS2-VASc score ≥ 2 in men or ≥ 3 in women and must also be considered when the score is ≥ 1 in men and ≥ 2 in women. However, factors that may modify the embolic or hemorrhagic risk of patients with cancer must be considered. Direct oral anticoagulants (DOAC) should be preferred over vitamin-K antagonists in nonvalvular AF. To evaluate these risks in more detail and address complex clinical scenarios, in 2020 the Spanish Society of Cardiology (SEC) Cardio-Oncology group created anticoagulation algorithms that facilitate assessment10 and the Spanish Thrombosis Group created an application to simplify the indication of anticoagulant treatment.11
HypertensionThe guidelines offer comprehensive recommendations on hypertension management in patients with cancer including a clear threshold for systolic blood pressure (BP) ≥ 180 mmHg or diastolic BP ≥ 110 mmHg when cancer medication should be deferred or stopped until BP control is adequate. Uncontrolled hypertension is acknowledged as a driver of cardiac toxicity and HF during treatment with anthracyclines, ibrutinib, or vascular endothelial growth factor inhibitors. The new algorithm emphasizes the use of angiotensin-converting enzyme inhibitors or ARB as the first option alone or in combination with dihydropyridine calcium channel blockers. This algorithm is highly useful in both cardiology and oncology/hematology practice. The new recommendation on using different thresholds for BP depending on cancer prognosis is based on expert opinion.
Thromboembolic eventsA new algorithm is presented which balances thromboembolic and bleeding risks to support difficult decisions in daily clinical practice.
For VTE secondary prevention, the first step (as in AF) is to assess thromboembolic risk, bleeding risk, treatment interactions, and patient preferences. When there is no contraindication to starting anticoagulation, the cardio-oncology guidelines recommend the use of LMWH or DOAC (both IA indications). LMWH will be the first option in unoperated gastrointestinal (GI) or genitourinary cancer, GI comorbidities or toxicity, severe renal dysfunction (creatinine clearance ≤ 15mL/min), DOAC major drug-drug interactions or platelet count<50 000/μL. In the remaining scenarios, LMWH or DOAC are recommended with the same class of recommendation, and patient-preference should be considered.
Use of half-dosage LMWH is assigned a class IIb indication if the platelet count is between 25 000 and 50 000/μL. In patients with a very high bleeding risk assessed by the presence of recent or active major bleeding, new or evolving intracranial lesions, or a platelet count of less than 25 000/μL, anticoagulation should be withheld even in the presence of VTE.
For primary prevention of VTE, the use of prophylactic LMWH is recommended in hospitalized patients (IB indication). In outpatients at high risk of VTE, LMWH, apixaban, or rivaroxaban may be used in the absence of contraindications (IIb B).
Despite the published evidence on the use of DOACs in patients with cancer and the fact that it is a more efficient and better tolerated treatment than LMWH, the lack of reimbursement in the Spanish health system is the main limitation to the use of these drugs in VTE or cancer-related thrombosis.
Other complicationsWhile there is little novelty on peripheral artery disease, there are new recommendations on how to monitor dasatinib-related pulmonary hypertension and when to stop the treatment (level of evidence C). Novelties on pericardial disease include recommendations on the treatment of immune checkpoint inhibitor-related pericarditis and the management of recurrent pericardial effusions with a pericardial surgical window or intrapericardial infusion of sclerosing agents.
End of cancer treatment assessmentThe cardio-oncology guidelines recommend cardiovascular assessment after cancer treatment. At the end of cancer therapy, cardiovascular risk assessment is circumscribed at 12 months after the last potentially cardiotoxic treatment. As a novelty, the document provides a risk classification and the corresponding clinical approach. The main objective of follow-up is to rule out CTRCD by using biomarkers and TTE and the frequency of follow-up will vary depending on the estimated risk. In our clinical setting, this follow-up can be eased using the SEC-Primaria program associated with an e-consult system that limits face-to-face hospital visits to the performance of TTE. 12,13
Since patients with low cardiorespiratory fitness have a poor prognosis, it is crucial to promote physical activity. CPET is recommended to assess the level of cardiorespiratory fitness in each patient and physical exercise is advised to all patients in cardiac rehabilitation programs. In our environment, it may be difficult to have a CPET for everyone, but selected patients may be offered a specific rehabilitation program. When CPET is not available, a simpler approach such as 30-second sit-to-stand and 6-minute walk tests can be used as an alternative to estimate functional capacity.14
Last, the withdrawal of HF drugs at the end of anticancer treatment in patients who had asymptomatic mild or moderate cardiotoxicity and who have fully recovered (normalized LVEF and biomarkers) is assigned a IIa C recommendation. This could be controversial in patients who had a significant drop in LVEF. It is true that this recommendation is limited to selected low-risk patients, but it is not clear whether the response of these patients may be similar or not to that of patients with HF and recovered LVEF, in which the ESC Heart Failure guidelines recommend withholding HF treatment due to the risk of cardiomyopathy recurrence.6,15 If HF treatment is withdrawn, the cardio-oncology guidelines highlight the importance of subsequent monitoring with biomarkers and TTE.
Follow-up of Long-term survivorsThe guidelines provide a specific risk classification for those patients who had an onco-hematological process during their youth or adulthood. The follow-up of these patients revolves around ruling out CTRCD in the first group and covers premature atherosclerosis in the second group. In both groups, the guidelines recommend complex image screening, which we could try to implement at least in high-risk patients.
It is important to emphasize the control of CVRF, which can be achieved with the SEC-Primaria program.5,12,13 Of note, the current long-term survivors referred to in this section do not have tomorrow's pharmacological treatments or radiological equipment, and consequently the recommendations in future guidelines could well change.
Special populationsThe document contains a nice figure showing the most frequent locations of primary and secondary cardiac tumors, reminding readers at a glance what to rule out in the different cardiac chambers.
There is a specific section dedicated to pregnancy, covering a highly complex area. Even if there are no relevant specific studies in high-risk cancer survivors, prepregnancy counseling and management during pregnancy and around delivery by a multidisciplinary pregnancy heart team is given a class I recommendation.
Rare diseases such as carcinoid or cardiac AL-amyloidosis are also discussed in this section. TTE is recommended for the detection of NP levels and/or clinical signs of carcinoid heart disease, and for surveillance every 3 or 6 months depending on the severity of cardiac involvement and clinical status. Cardiac amyloidosis has even more impact in clinical practice due to recent therapeutic progress. TTE and CMR, NP and troponin are recommended for the diagnosis of amyloid light-chain cardiac amyloidosis in patients with plasma cell dyscrasia (class IC). Endomyocardial biopsy is assigned a IIa class recommendation.
The section ends with a highly practical recommendation on the management of patients with a cardiac implantable electronic device located in the radiotherapy treatment beam and of those located outside the radiotherapy treatment volume.
Nursing in cardio-oncologyThis is probably the only section that the authors of the current document find deficient. Although there are some sections that address lifestyle habits, physical exercise, diet, etc, and that the “specification of roles of different health care professionals, including nurses and pharmacists” is considered a gap in evidence, we would have liked an explicit section on nursing in cardio-oncology. The guidelines highlight the importance of the MDT and communication with patients, encouraging their involvement and participation in their management, and this task should be led by nurses. Unhealthy lifestyles and poor therapeutic adherence encourage CVRF, CVD, and cardiotoxicity. Integrated general and specific self-care support programs in different oncological processes have shown a marked improvement in the perceived quality of life and level of self-care of affected individuals, improving their degree of autonomy and overall outcomes. Structured counseling should be provided to encourage healthy behaviors and the identification and strict CVRF control (healthy diet, smoking cessation, regular exercise, and weight control) before, during and after treatment, the appropriate use of available resources, and clinical follow-up to rule out symptoms and signs suggestive of cardiotoxicity.16
In the SEC cardiovascular risk assessment consensus document,5 nurses play a fundamental role in cardiotoxicity prevention, with cost-effective strategies such as the identification, control, and monitoring of CVRFs before, during and after treatment, early detection of warning signs and/or symptoms, and promotion of a healthy lifestyle.
Quality indicators for cardio-oncologyQuality improves when it is monitored; however, best practices should be based on those actions that provide the greatest benefit, and the scant clinical evidence in cardio-oncology makes it difficult to strictly establish quality indicators in this field at present.
The guidelines recognized the importance of developing tools for measuring quality, and another task force is preparing a dedicated document that will be published soon. Many parameters may be used to define quality in different domains and all should be at least visible; however, considering the existing level of evidence, it would be better to at least highlight a few truly relevant and amenable parameters for easy audits and benchmarking.
We would expect the inclusion of simple data, such as a) a cardio-oncology unit/team officially structured, organized, and recognized at each hospital/site; b) the number of cases with at least 1 visit (at baseline) among the total number of patients with a new cancer diagnosis who will be prescribed potentially cardiotoxic therapies; and c) the number of patients with HF or another major cardiovascular event/year with and without a recorded previous visit by the MDT.
Linked to quality, the national and international cardiology societies may provide guidance and audits at a local level. The SEC is currently developing a program to help the organization and certification of cardio-oncology units.
CONCLUSIONSThe 2022 ESC cardio-oncology guidelines provide an excellent reference to support all professionals involved in the assessment and treatment of patients with cancer. However, many centers will not be able to apply the huge number of new recommendations, and therefore we should probably focus on high-risk and very high-risk patients until resources for cardio-oncology units are increased. Because of the scarce number of clinical trials and meta-analyses published in this field, most recommendations are level of evidence C. We must encourage research in cardio-oncology to improve the evidence in future guidelines. From the Cardio-Oncology group of the Spanish Society of Cardiology and the SEC Working Group for the 2022 ESC cardio-oncology guidelines, we also encourage the use of our previous tools and local consensus documents to apply the new recommendations and improve the cardiovascular care of patients with cancer. Finally, we are grateful to the authors of the ESC cardio-oncology guidelines for the creation of an excellent document as well as the accompanying tools to facilitate its use and implementation.
FUNDINGNone.
CONFLICTS OF INTERESTThe conflict-of-interest declaration documents of all authors can be seen in the .
SEC Working Group for the 2022 ESC guidelines on cardio-oncology
Pedro Moliner (coordinator), Pilar Mazón (coordinator), Alberto Cordero, Concepción Fernandez, José Luis López Sendón, Ana Martín García, Amparo Martinez-Monzonís, Cristina Mitroi, Milagros Pedreira, Nuria Vallejo, José Luis Zamorano, Eduardo Zatarain-Nicolás.
SEC Guidelines Committee: Rut Andrea, Pablo Avanzas, Gemma Berga, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, Pilar Mazón, Domingo Pascual, Juan Sanchis, José M. de la Torre, David Vivas, José L. Ferreiro (president).
Supplementary data associated with this article can be found in the online version, at http://dx.doi:10.1016/j.rec.2022.12.004
The names of all the authors of the article are listed in alphabetical order in Appendix A.
See related article: https://secardiologia.es/cientifico/guias-clinicas/cardio-oncologia/13910-2022-esc-guidelines-on-cardio-oncology-developed-in-collaboration-with-eha-estro-and-ic-os.
Corresponding author:.
Email addresses: pilarmazon@yahoo.es (P. Mazón); pmolinerborja@gmail.com (P. Moliner).