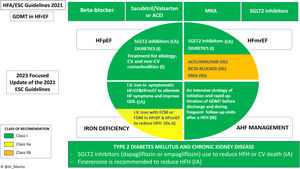
The European Society of Cardiology (ESC) published their update1 to include new recommendations based on scientific evidence that emerged within the last heart failure (HF) guidelines in 2021 and on March 31, 2023.2 Of note, only findings affecting class I or IIa recommendations have been incorporated and are related to the primary objectives of clinical trials, mostly HF hospitalization or cardiovascular death. Undoubtedly, this update is mainly justified by the benefit of sodium-glucose co-transporter 2 inhibitors (SGLT2i) in patients with mildly reduced (HFmrEF) and preserved (HFpEF) left ventricular ejection fraction (LVEF), as well as hospitalized patients with HF. The rest of the changes mainly strengthen or upgrade previous recommendations, except for the novelty of finerenone, a new selective mineralocorticoid receptor antagonist (MRA) (figure 1).
Central illustration. Summary of the new recommendations in the 2023 update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. ACE-I, angiotensin-converting enzyme inhibitor; AHF, acute heart failure; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CV, cardiovascular; ESC, European Society of Cardiology; FCM, ferric carboxymaltose; FDM, ferric derisomaltose; GDMT, guideline-directed medical therapy; HF, heart failure; HFA, Heart Failure Association; HFH, heart failure hospitalization; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; I.V, intravenous; MRA, mineralocorticoid receptor antagonist; QOL, quality of life; SGLT2, sodium-glucose co-transporter 2.
Undoubtedly, the greatest weight of evidence that has justified this review of the 2021 guidelines has been the studies with SGLT2i-dapagliflozin and empagliflozin- in the phenotypes of chronic HF with HFmrEF (LVEF 41%-49%) and HFpEF (LVEF ≥50%). The EMPEROR-Preserved3 and DELIVER4 trials had positive and robust results in these phenotypes, leading to the highest class of recommendation (I) and level of evidence (A). In relation to this recommendation, several aspects should be highlighted. Firstly, the benefit in all subgroups of patients, which in the case of dapagliflozin (DELIVER) also included patients with recovered LVEF, a subgroup not previously studied. Secondly, both clinical trials and the aggregated meta-analysis of both populations showed that the main benefit was explained by a lower risk of HF hospitalization, around 26%, whereas no significant reduction in CV mortality alone was achieved in these population phenotypes.5 The Task Force, considering the significant reduction in the primary endpoint, establishes the recommendation (IA) for the prevention of HF hospitalizations or cardiovascular mortality separately for patients with HFmrEF and for patients with HFpEF. This separation is a consequence of the current definitions of HF based on LVEF phenotypes; however, the overall results obtained with SGLT2i should lead to overcoming the dichotomies of these phenotypes. Indeed, the prespecified pooled analysis of dapagliflozin across the entire LVEF spectrum (DAPA-HF+DELIVER) found a reduction in each individual endpoint: HF hospitalization, cardiovascular mortality, and total mortality.6 Therefore, we are in a paradigm shift, and for the first time, the recommendation of a pharmacological group, SGLT2i, could have been established for patients with HF (IA), irrespective of LVEF. Likewise, we should highlight the rapid implementation of SGLT2i in clinical practice, ahead of these recommendations, which is explained by the safety and ease of use of a single daily dose, with no need for additional up-titration.
Hospitalized patient with decompensated HFDiuretic combinationsThis update finds value in 2 recent clinical trials that have evaluated the safety and efficacy of acetazolamide (ADVOR7) and hydrochlorothiazide (CLOROTIC8) combined with loop diuretics in hospitalized patients with acute HF. In both studies, acetazolamide (500mg) and oral hydrochlorothiazide (25-100mg according to glomerular filtration rate) significantly reduced signs of congestion at 72hours after admission, which was the primary endpoint in both studies. Additionally, in the ADVOR study, acetazolamide decreased the mean length of stay by 1 day, a benefit that has not been demonstrated by any other diuretic to date. However, none of these strategies achieved improvement in secondary endpoints, which included clinical events such as mortality or rehospitalization for HF. Moreover, in the CLOROTIC study, hydrochlorothiazide showed a higher rate of worsening renal function and hypokalemia compared with placebo. Therefore, although the impact on decongestion of both diuretic therapies is recognized, the Task Force concludes that more data on safety and efficacy in terms of clinical events are needed before establishing any class of recommendation for combined diuretic strategies added to furosemide as a first step. Therefore, the diuretic algorithm proposed in previous guidelines is maintained, which advocates increasing the dose of loop diuretic if there is persistent congestion and poor diuretic response.
SGLT2 inhibitorsThe update incorporates new evidence with SGLT2i in patients hospitalized for HF, mainly derived from the EMPULSE study.9 Early initiation of empagliflozin, within the first 72hours after admission and once hemodynamic stability is achieved, was safe and showed efficacy by improving a hierarchical composite outcome (win-ratio method) including mortality, new HF hospitalizations, and quality of life at 90 days. These results did not vary according to LVEF or diabetic status and support the safety of early initiation during hospitalization. Simultaneously with the presentation of this update, the results of dapagliflozin initiated within the first 24hours after admission were also presented, reinforcing the safety of early initiation of SGLT2i in this clinical setting. In addition, previous results from the DELIVER and SOLOIST studies, which also included hospitalized or recently hospitalized patients, further support the key role of SGLT2i in the setting of worsening HF.1 Therefore, considering all the current evidence, the update positions SGLT2i as the pharmacological mainstay for both hospitalized patients with acute HF and outpatients with chronic HF, irrespective of LVEF.
Intensive pharmacological optimizationAlthough already included in the previous guidelines, the publication of the results of the STRONG-HF study10 has led to emphasize on the need to initiate and intensively optimize evidence-based treatments in hospitalized patients. For this aim, the Task Force establishes a specific class I recommendation (level of evidence B) for an intensive strategy prior to discharge and consisting of close follow-up after discharge. In the STRONG-HF study, nonoptimized patients were randomized to a conventional vs intensive strategy, which included rapid optimization during the 2 days prior to discharge to at least half doses, and full dosing during the first 2 weeks after discharge. The intensive strategy was safe and led to a higher likelihood of receiving full doses of disease-modifying therapies, which was associated with a significantly lower rate of the primary endpoint at 180 days (rehospitalization for HF or death from any cause). Although these positive results were achieved with the classic triple therapy for HFrEF (beta-blockers, ACE-I or ARNI, and MRA), their results can be extrapolated to the current quadruple therapy including SGLT2i. Indeed, the recommendation is aimed at HF patients and evidence-based disease-modifying therapies, without considering LVEF phenotypes.
Transition and care organizationThe previously discussed recommendation, intensive pharmacological optimization, can only be implemented if considered within the process of “transition” from the inpatient to the outpatient setting. The transition represents an organized pathway, which must be structured in a multidisciplinary and comprehensive manner. The 2021 guidelines already highlighted the importance of these predischarge and postdischarge phases, with a relevant role for an early visit at 7 to 14 days after discharge, giving it a class I recommendation, although the evidence supporting it was expert opinion (C). The STRONG-HF trial10 included weekly visits for the first 3 weeks and at 6 weeks postdischarge; therefore, this update includes in the recommendation for an intensive strategy not only rapid therapeutic optimization, but also the need for frequent visits during the first 6 weeks, with class I and upgraded evidence to B. As stated in the text of the document, these visits should include reassessment of congestion and monitoring of vital constants, natriuretic peptide levels, potassium, and renal function. In this regard, the Spanish Society of Cardiology was a pioneer in 2019, releasing a document that included a call to action for organizing the transition period and specific recommendations for this purpose. In the 2021 update of this document, promoted by the Spanish Heart Failure Association,11 it is also recommended to adapt the transition process to the local characteristics, to use written documents, and to involve all professionals participating in the care of HF patients, in particular nurses and primary care physicians. A multidisciplinary approach is the only way to incorporate close follow-up after discharge and to overcome the barriers for repeated visits during this period, in order not only to intensively optimize therapies but also to provide other relevant elements such as education, adherence, and early detection of complications.
ComorbiditiesChronic kidney diseaseThis update incorporates new recommendations to prevent HF in patients with CKD and type 2 diabetes, which are populations with a particularly high risk of developing HF. Once again, new evidence has mainly arisen from SGLT2i with 2 clinical trials assessing the impact of dapagliflozin (DAPA-CKD) and empagliflozin (EMPA-KIDNEY) in patients with established CKD (nonsevere, estimated glomerular filtration rate >20-25mL/min/1.73 m2), with or without diabetes, and a small percentage (10%-11%) with a prior diagnosis of HF.1 Both trials showed a reduction in the risk of progression of kidney failure or cardiovascular death, and DAPA-CKD also showed a significant reduction in hospitalization for HF or cardiovascular death. However, the Task Force based the new recommendation on a meta-analysis including all pivotal clinical trials with SGLT2i and not only the results of those 2 specific trials in CKD.1 In this meta-analysis, in the absence of previous HF, the prevention of HF hospitalization or cardiovascular death was only significant when patients with concomitant diabetes and CKD were considered together.12 This finding has led to a class I recommendation (evidence level A) for SGLT2i in diabetic patients with CKD to prevent HF hospitalizations or cardiovascular mortality, even though diabetes was not an inclusion criterion in the DAPA-CKD and EMPA-KIDNEY trials. The other novelty is the recommendation for finerenone, a new selective MRA, based on a prespecified pooled analysis (FIDELITY) that included 13 026 diabetic patients with CKD followed for a median of 3 years in the FIDELIO-DKD and FIGARO-DKD trials.12 In this pooled analysis, finerenone was associated with a 14% reduction in a cardiovascular composite endpoint (CV death, nonfatal stroke, nonfatal myocardial infarction, and HF hospitalizations) and, importantly for this recommendation, with a 22% reduction in HF hospitalizations. As a result, as for SGLT2i, finerenone is recommended as a class I (evidence level A) treatment in diabetic patients with CKD to prevent HF hospitalizations.
Iron deficiencyThis update strengthens the indication for intravenous iron therapy to a class I recommendation (previously IIa), with level of evidence A, for the improvement of symptoms and quality of life in patients with HFrEF or HFmrEF. This means that the cutoff of LVEF <45% in the 2021 guideline is increased to <50% in this update. In addition, ferric derosimaltose is also included together with ferric carboxymaltose, both formulations with a class IIa recommendation and level A evidence (previously B), for the prevention of HF hospitalizations, not only in recently hospitalized patients but also in all HF scenarios. For the upgrade in this recommendation, the positive results from the IRONMAN-HF study, with iron derosimaltase in patients with chronic HF and LVEF <45%, and the results of several meta-analyses were considered.1 Of note, the benefit in the primary endpoint of HF hospitalization is mainly supported by censoring the analysis to the pre-COVID period in a prepandemic sensitivity analysis. However, simultaneously with this update, the results of the HEART-FID trial13 were published, in which the use of iron carboxymaltose in outpatients with iron deficiency and HFrEF showed no significant improvement with respect to a hierarchical endpoint including death, HF hospitalization, and distance in the 6-minute walking test. Also simultaneously, a meta-analysis of the 3 studies with iron carboxymaltose was published,14 which identified a beneficial effect in terms of total and HF-related hospitalizations, but not in terms of mortality, which would be in line with the current recommendation, mainly based on meta-analyses results. However, the new results from the HEART-FID trial could lead to revision of the level of evidence of this recommendation in the future.
IMPLICATIONS FOR THE SPANISH HEALTH CARE SYSTEMThis update strengthens several areas of improvement in the care of our HF patients. On the one hand, from a pharmacological point of view, it strengthens the implementation of SGLT2i in the entire spectrum of HF, and of HF prevention in diabetic patients with concomitant CKD. On the other hand, from a disease management perspective, it emphasizes the need to organize the care of hospitalized patients with HF, integrating predischarge and postdischarge periods and facilitating the coordinated participation of specialists, nursing, and primary care. In this context, the update reinforces the recommendation to initiate and optimize those disease-modifying therapies intensively, and to avoid therapeutic inertia in any HF scenario. To achieve this objective efficiently, as recently reported, the use of new modes of physician interaction such as e-consult may be useful.15
GAPSThe first gap is acknowledged by the guidelines themselves, indicating that the Task Force considered changing the definition of “preserved LVEF” to “normal LVEF”. This change will probably occur in the next guidelines. However, beyond this, it would be appropriate to move away from LVEF-based phenotypes and evolve toward the concept of HF as a global disease and the use of personalized treatments based on imaging and/or blood biomarkers, as is already the case in oncology.
Another relevant gap is the delay in guideline updates in relation to the publication of new evidence, which should prompt a reconsideration of their frequency. In fact, some of the updates are already reflected in clinical practice. A good example is the new recommendations for SGLT2i, which are based on publications from 2 years ago, just prior to the release of the previous guidelines in 2021. In the past, guidelines served as a starting point for translating new evidence into recommendations that could be applied in clinical practice. Nowadays, the guidelines represent a scientific endorsement for evidence that, due to its weight, has already permeated clinical practice. Perhaps we are now overcoming the main limitation of past guidelines, which was the slow translation of evidence into real-world practice. However, while this applies to medications, it is not the case for some other class I recommendations, such as cardiac rehabilitation, which is not widely implemented in our health system. In addition, as mentioned in this document, some of the recommendations are affected by simultaneous publications released during the ESC Congress, which renders the guidelines outdated at the time of publication. This limitation does add value to the present comments from the guidelines committee of the Spanish Society of Cardiology, which should serve to partially address this limitation.
FUNDINGNone.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
CONFLICTS OF INTERESTThe conflict of interest declaration documents of all authors can be seen in the Supplementary data.
SEC Working Group for the 2023 update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Domingo Pascual-Figal (coordinator), Nicolás Manito-Lorite (coordinator), Antoni Bayes-Genis, Esther Calero-Molina, Marta Cobo, Juan Delgado, Inés Gómez-Otero, Julio Núñez-Villota, Alejandro Recio.
SEC Guidelines Committee: José Luis Ferreiro (president), Pablo Avanzas (secretary), Rut Andrea, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, María Antonia Martínez Momblan, Pilar Mazón, Domingo Pascual-Figal, Juan Sanchis, José María de la Torre Hernández, David Vivas.
The names of all the authors of the article are listed in alphabetical order in Appendix A.
See related content: https://secardiologia.es/cientifico/guias-clinicas/insuficiencia-cardiaca-y-miocardiopatia/14537-2023-focused-update-of-the-2021-esc-guidelines-for-the-diagnosis-and-treatment-of-acute-and-chronic-heart-failure.
Corresponding authors.
Email addresses:dpascual@um.es (D. Pascual-Figal); nml@secardiologia.es (N. Manito-Lorite).