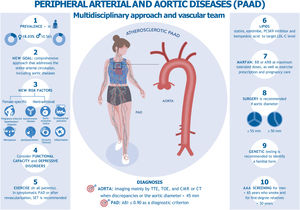
The 2024 update of the ESC clinical practice guidelines on peripheral arterial disease (PAD) also includes aortic diseases, updating the 2017 and 2014 guidelines. This update has been endorsed by the European Association for Cardio-Thoracic Surgery (EACTS), the European Reference Network on Rare Multisystemic Vascular Diseases (VASCERN), and the European Society of Vascular Medicine (ESVM).1 In addition to input from health care professionals from multiple disciplines, patients also contributed to the update through patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs) included in the guideline recommendations figure 1.
Central illustration. Peripheral arterial and aortic diseases, multidisciplinary approach, and vascular team. AAA, abdominal aortic aneurysm; ARB, angiotensin receptor blocker; BB, beta-blocker; CCT, cardiovascular computed tomography; CMR, cardiovascular magnetic resonance; PAD, peripheral arterial disease; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; SET, supervised exercise training; TTE, transthoracic echocardiography; TOE, transesophageal echocardiography.
This new document unifies the concept of peripheral arterial and aortic disease (PAAD) as an entity characterized by high prevalence and significant morbidity and mortality. Two fundamental concepts emerge: shared decision-making with patients and a multidisciplinary approach to managing complex patients or procedures in high-volume and high-experience centers.
WHAT IS NEW IN THE GUIDELINES?Evaluation of peripheral arteries and aortaAs a novel addition, it is recommended to adopt a comprehensive approach that addresses the entire arterial circulation. This includes an objective assessment using specific questionnaires or tests to evaluate functional capacity and depressive disorders.
The guidelines review the various imaging modalities available for assessing the thoracic and abdominal aorta. Detailed discussions on how to perform measurements using different techniques are provided without altering the recommendations (table 1).
Thoracic and abdominal aortic aneurysms: aetiology, screening and diagnostic methods
| Methods | TAA | Abdominal aortic aneurysm | |
|---|---|---|---|
| Aetiology | Root and ascending aortaHTADBAVSporadic TAAAtherosclerosis | Descending aortaAtherosclerosisAortitis (infectious or not)TraumaCoarctation | Media degenerationInflammationGenetic disordersInfectionAtherosclerosis |
| Screening | TTE | DUS | |
| Diagnostic | TTE or TOE plus CCT or CMR | DUS or CEUS, CCT or CMR | |
BAV, bicuspid aortic valve; CCT, cardiovascular computed tomography; CEUS, contrast-enhanced Doppler ultrasound; CMR, cardiovascular magnetic resonance; DUS, Doppler ultrasound; HTAD, heritable thoracic aortic disease; TAA, thoracic aortic aneurysm; TOE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Screening for PAD is not systematically recommended, except in patients older than 65 years with risk factors (IIaC). It is also proposed that diabetic patients or those with chronic kidney disease who have a normal ankle-brachial index (ABI) at rest should undergo a toe-brachial index test.
Multisite artery disease (MAD) is defined as the presence of atherosclerosis in 2 or more vascular beds. While it is not proposed to be actively identified, MAD is recognized as having added value for the risk reclassification of patients with intermediate to high risk. This allows the identification of patients who may benefit from more intensive preventive treatments or strategies that combine antiplatelet therapy with low-dose anticoagulation (COMPASS strategy).
For abdominal aortic aneurysm screening, duplex ultrasound (DUS) (IA) is recommended for men older than 65 years who smoke and for first-degree relatives older than 50 years (IC). Opportunistic abdominal aortic aneurysm screening is also considered in patients with PAD who undergo DUS. Interestingly, cardiologists may also consider screening men older than 65 years and women older than 75 years who undergo echocardiography, even in the absence of risk factors (IIaB).
Optimal medical treatmentOptimal management of PAAD involves a synergistic approach that combines pharmacological therapy with lifestyle modifications. Key lifestyle changes include: a) adhering to a Mediterranean diet, b) engaging in regular physical activity to improve functional capacity, and c) quitting smoking to reduce disease progression. Patient education and structured support programs also play a critical role in promoting self-care and improving treatment adherence, which further enhances clinical outcomes.
The pharmacological management of PAAD focuses on addressing the underlying pathophysiology, reducing cardiovascular (CV) risk, and slowing the progression of the disease. Pharmacological therapy includes the following:
Antithrombotic therapyIn patients with asymptomatic PAD, the available evidence does not support routine treatment with antiplatelet drugs. However, aspirin (75-100mg) may be considered in diabetic patients in the absence of contraindications (IIbA). In patients with symptomatic PAD, a single antiplatelet agent, either aspirin or clopidogrel, remains the preferred long-term treatment (IA). As a novelty, the guidelines now recommend the combination of rivaroxaban (2.5mg twice a day) and aspirin (100mg once a day) in patients with PAD who have high ischemic risk, high-risk comorbidities (IIaA), or who have undergone lower-limb revascularization (IIaB), provided that their risk of bleeding is not elevated.
Antihypertensive therapySystolic blood pressure should be treated to a target range of 120 to 129mmHg, although personalized targets may be required in older or frail patients. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers should be considered as first-line antihypertensive agents. In many cases, combination therapy—including diuretics, beta-blockers, and/or calcium channel blockers—is often required to achieve optimal blood pressure control.
Lipid-lowering therapyIn patients with atherosclerotic PAAD, the therapeutic goal is to reduce low-density lipoprotein cholesterol (LDL-C) by ≥ 50% and achieve an LDL-C target of <55mg/dL. Statins are recommended for all patients with PAD. If LDL-C goals are not met with statin monotherapy, adding ezetimibe or PCSK9 inhibitors is advised. In patients with statin intolerance and who fail to achieve their LDL-C target with ezetimibe alone, incorporating bempedoic acid is recommended, either as monotherapy or in combination with a PCSK9 inhibitor.
Antidiabetic therapyScreening for diabetes or prediabetes in PAAD patients is essential, as optimized treatment significantly influences clinical outcomes. It is recommended to target a glycated hemoglobin level of 7%, with higher thresholds for those with limited life expectancy. The guidelines recommend prioritizing sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists that have demonstrated CV benefits. These should be preferred over agents without proven CV benefit or safety, followed by agents with demonstrated CV safety.
Exercise therapyOptimal medical treatment in patients with symptomatic PAD includes supervised exercise training (SET), which has the highest level of evidence (Ia). SET is safe and improves treadmill walk distance (both peak and pain-free), health-related quality of life, and cardiorespiratory fitness. However, exercise has not been shown to improve ABI. SET programs should be conducted at least 3 times a week for 30 to 60minutes over a minimum of 12 weeks.
In patients with symptomatic PAD who experience impaired PAD-related quality of life after 3 months of optimal medical treatment and exercise therapy, revascularization may be considered (IIbB). In those who undergo endovascular revascularization, SET is recommended as an adjuvant therapy (IA) due to its significant improvements in walking performance, health-related quality of life, and reduction in future revascularization needs.
When SET is not available, home-based exercise training or alternative modalities—such as strength training, arm cranking, cycling, and combinations of these—should be recommended (IIaA). Evidence regarding the best intensity for exercise training is less robust. While vigorous exercise is less effective than light-to-moderate intensity in improving walking distance, it offers greater benefits for cardiorespiratory fitness.2,3 For this reason, vigorous intensity exercise has a IIaA recommendation.
Training programs should start at a low-to-moderate intensity and gradually progress to vigorous exercise if well tolerated. This approach allows assessment of patient response and adherence while minimizing complications.
Genetic and congenital disease of the aortaGiven the complexity and scarcity of testing, the guidelines recommend that individuals with suspected genetic aortic disease be evaluated in specialized centers with expertise in assessing patients and their first-degree relatives for genetic testing. The guidelines also provide a specific screening algorithm for thoracic aortic disease. A comprehensive evaluation of the entire aorta and other vascular regions is recommended in patients with hereditary thoracic aortic disease. Both genetic testing and imaging—mainly through transthoracic echocardiography, cardiovascular magnetic resonance, or cardiovascular computed tomography if the aortic root/ascending aorta is not well visualized—are essential for diagnosing hereditary thoracic aortic disease in patients and family members. In patients with a strong suspicion of an underlying genetic defect but no identified genetic cause, a genetic reassessment in 3 to 5 years should be considered.
As a novelty, specific recommendations are provided for evaluating imaging or surgery in patients with Turner syndrome, Ehlers-Danlos syndrome, and Loeys-Dietz syndrome. For Marfan syndrome, the main recommendations focus on imaging follow-up, medical treatment (beta-blockers or angiotensin receptor blockers at maximum tolerated doses), exercise prescription, and pregnancy care.
The recommendation to adopt a new international consensus nomenclature and classification for patients with bicuspid aortic valve and aortopathy, replacing various competing nomenclatures, is a positive development. Surgery for bicuspid aortopathy continues to be recommended when the maximum aortic diameter is ≥ 55mm. As a new recommendation, surgery for the root phenotype (IB) of bicuspid aortopathy is advised when the maximum aortic diameter is ≥ 50mm.
Polyvascular peripheral artery disease and peripheral artery disease in patients with cardiac diseasesPolyvascular diseaseIn the new guidelines, the term MAD has been replaced by polyvascular disease (PVD). This entity is defined as the simultaneous presence of clinically relevant obstructive atherosclerotic lesions in at least 2 major arterial territories. It is common in patients with atherosclerosis and independently increases the risk of major CV events. While the clinical benefit of systematic screening for PVD is questionable, it can be useful to identify high-risk patients and guide therapeutic decisions. In this context, a combination of rivaroxaban (2.5mg twice a day) and aspirin (100mg once a day) should be considered in patients with symptomatic PVD in at least 1 territory who do not have a high bleeding risk (IIaA). In patients with PVD, a reduction in LDL-C of ≥ 50% from baseline, aiming for a target of <1.4 mmoL/L (< 55mg/dL), is recommended, together with proactive management of all modifiable risk factors through lifestyle changes and pharmacological treatment.
Regarding the screening for carotid stenosis by DUS in stable patients scheduled for coronary artery bypass grafting who have experienced a transient ischemic attack or stroke within the past 6 months and have not undergone carotid revascularization, this indication has been downgraded to class IIa, level B.
PAD and heart failure, atrial fibrillation, and aortic stenosisThe guidelines highlight the significant impact of the association between heart failure or atrial fibrillation and PAD on CV events and mortality. In these patients, intensive risk-factor modification strategies and optimization of heart failure therapy are warranted. Obviously, full oral anticoagulation is recommended in those with atrial fibrillation and a CHA2DS2-VASc score ≥ 2 (IC).
Finally, the guidelines stress the importance of assessing the iliofemoral system in patients undergoing transcatheter aortic valve implantation (IB).
CONSEQUENCES OF THE IMPLEMENTATION OF THE GUIDELINESInterventional treatmentSymptomatic peripheral artery diseaseThe new guidelines recommend that patients with symptomatic PAD and iliac lesions be treated with balloon angioplasty, with or without stenting, in the external iliac arteries, or with primary stenting in the common iliac arteries (IIaB). The European Society for Vascular Surgery 2024 guidelines recommend the use of self-expanding bare metal stents over balloon-expandable stents due to their lower risk of restenosis and target lesion revascularization when treating iliac artery lesions (IIbB).4 However, in patients with extensive aorto-iliac atherosclerotic lesions (TASC II C/D lesions), covered stent placement may be preferred over bare metal stents because of higher patency rates (IIbB). Additionally, the current guidelines recommend drug-eluting treatments as the first-choice strategy for femoro-popliteal lesions (IIaA). In patients with acute limb ischemia, the guidelines recommend following revascularization with dual antiplatelet therapy or rivaroxaban (2.5mg twice a day) and aspirin (100mg once a day) if they are not on anticoagulation for other reasons (IIaC).
Abdominal aortic aneurysmRecommendations for the surveillance and treatment of patients with abdominal aortic aneurysm establish either a long or limited life expectancy at 2 years. Another novelty is the recommendation for elective repair in patients with saccular aneurysms measuring ≥ 45mm (IIbC).
Thoracic aortic aneurysmPatients with dilatation of the tubular ascending aorta, tricuspid aortic valve, and low predicted surgical risk should be considered for ascending aortic replacement when the maximum diameter exceeds 52mm (IIaB), while surgery is recommended for diameters of ≥ 55mm (IB). In patients undergoing surgery for tricuspid aortic valve disease who also have concomitant dilatation of the aortic root or ascending tubular aorta and low predicted surgical risk, ascending aorta or root replacement should be considered when the maximum diameter is at least 45mm; otherwise, the threshold is 50mm. Preoperative evaluation and initial follow-up of patients are shown in table 2.
Preoperative evaluation and initial follow-up of patients
| Perioperative management of patients with surgically treated aortic root and ascending aortic aneurysm | |||||
|---|---|---|---|---|---|
| PRE-SURGERY | TTE and TOEAortic valve assesment | STJ dilatation | STJ and aortic root dilatation | Annulus dilatation | Aortic valve disease |
| CCT or CMR Annulus/root/ascending aorta assessment | Supracommisural tubular graft | Aortic valve sparing | Annuloplasty | Bentall procedure | |
| BEFORE DISCHARGE | TTEInmediate cardiac complications | CCT or CMRRule out aortic complications | |||
| 1 MONTH | TTEAortic valve function and gradientsAortic root and proximal ascending aorta assessment | ||||
| FROM1 YEAR | TTEAortic valve function and gradientsAortic root and proximal ascending aorta assessment | CCT or CMREvaluation of aortic disease progression and rule out complications | |||
| Bioprosthesis:TTE/year | Mechanical prosthesis or native valve:TTE based on gradients, symptoms or residual AR | Aorta totally repaired:Follow-up at 2 years and then every 5 years | Aorta not fully repaired:Link with aneurysm follow-up algorithm | ||
AR, aortic regurgitation; CCT, cardiovascular computed tomography; CMR, cardiovascular magnetic resonance; STJ, sinotubular junction; TOE, transesophageal echocardiography; TTE, transthoracic echocardiography.
For the treatment of aortic arch, open repair remains the gold standard for symptomatic patients with aortic arch aneurysms (IC) or asymptomatic patients with low operative risk and an arch diameter≥ 55mm (IIaB). Hybrid or endovascular treatment is reserved for patients who meet the criteria for intervention but are at high surgical risk (IIbC).
Endovascular treatment is preferred over open repair in patients without hereditary thoracic aortic disease who have unruptured descending thoracic aorta aneurysms when elective repair is indicated (diameter >55mm) and anatomy is suitable (IB). Additionally, in patients with degenerative thoracoabdominal aortic aneurysms measuring≥ 60mm and with suitable anatomy, endovascular repair using fenestrated and/or branched endografts should be considered in experienced centers (IIaB).
Regarding new developments in acute aortic syndrome, in patients with complicated type B aortic dissection, the level of indication (IA) is raised both for emergency intervention and for the choice of endovascular repair as first-line therapy.
Patient participation and careThe ESC 2024 Guidelines represent a significant development in the care of patients with PAD, emphasizing a holistic and personalized approach. A key aspect of these guidelines is patient education and psychosocial support, which aim to promote lifestyle changes such as smoking cessation, a healthy diet, and regular physical activity. These elements are essential for improving long-term outcomes and preventing disease progression.5 The education outlined in this guideline is tailored to the individual needs of each patient, taking into account their health literacy and possible cultural barriers. This enhances treatment adherence and empowers both patients and caregivers6 (IA).
One of the major innovations in the guidelines is the incorporation of digital technologies, such as mobile applications and personalized risk calculators (IIaB). These tools facilitate secondary prevention and remote monitoring, optimizing adherence to treatment by enabling more efficient and accessible patient management. The guidelines highlight that these digital tools show promise in improving patient outcomes, but the level of evidence indicates they are still relatively new in terms of widespread clinical application.
Additionally, active patient participation in shared decision-making has gained prominence, as it fosters patient self-efficacy and reduces the risk of hospitalization. This patient-centered approach also improves quality of life. The guidelines introduce PREMs and PROMs as essential tools for evaluating both the patient's treatment experience and their functional, mental, and social status (IIaB).
LIMITATIONSEpidemiology and risk factorsThe guidelines continue to recommend assessing the risk of CV disease by evaluating traditional risk factors, such as SCORE2 and SCORE OP in Europe and ASCVD in the United States. While these scales are referenced, along with the risk of PAD associated with various traditional and nontraditional risk factors, the section lacks sufficient detail, which is crucial for disease prevention. Additionally, there is no mention of the QR4 scale, which incorporates 7 new risk factors: chronic obstructive pulmonary disease, learning disabilities, Down syndrome, and cancers of the blood, lung, mouth, and brain. This highlights the impact of other significant diseases on heart health. The QR4 scale also identifies specific factors in women's health that predict future CV risk, such as complications from high blood pressure during pregnancy and postpartum depression. Overall, the QR4 scale offers a more accurate estimate of CV disease risk than ASCVD or SCORE.
DiagnosisABI measurement is recommended as the first-line noninvasive test for screening and diagnosing PAD. In patients with exertional limb pain relieved by rest and a resting ABI >0.90, exercise testing with postexercise ABI measurements may be an alternative, but no specific recommendation is made, unlike in the American guidelines, where it is a type I recommendation.7
Toe-brachial index, toe pressure, and transcutaneous oxygen pressure measurements play a significant role, especially in patients with noncompressible ankle arteries or an ABI> 1.40. However, sensitivity and specificity vary widely, and situations that may affect their value are common. Therefore, this recommendation should be applied with caution, particularly in doubtful cases.
Vascular teamA multidisciplinary approach is fundamental in the treatment of PAD, with nursing playing a prominent role in coordinating care and providing continuous follow-up (IA). However, the guidelines do not address the role of advanced practice nurses, whose involvement could significantly enhance the effectiveness of interventions in patients with complex chronic diseases like PAD.8 These nurses are capable of performing advanced assessments, managing long-term follow-ups, and actively participating in clinical decision-making, thereby optimizing clinical outcomes.
The guidelines also omit care coordination and the important role of general practitioners. Additionally, the recommendations regarding the urgency of referrals to surgery are not explained.9 The guidelines include as a recommendation that e-cigarettes may be considered.
SUMMARYThese guidelines are extensive, detailed, and include great strengths and clinical implications, but also some gaps.
StrengthsThe 2024 ESC clinical practice guidelines cover PAD and aortic diseases, highlighting their greater prevalence in women, even in the central illustration. As a novelty, the guidelines recommend addressing the entire arterial circulation and include specific questionnaires or tests for functional capacity and depressive disorders. Optimal management involves risk factor control, pharmacological therapy, lifestyle modifications, patient education, and exercise as a key component. In patients with symptomatic PAD, SET is recommended. Additionally, there are new surgical recommendations for abdominal and thoracic aortic aneurysms and for screening.
GapsCare coordination is not included, nor is the important role of general practitioners. Equally, advanced practice nurses are not mentioned. Recommendations on the urgency of referrals for surgery are not explained. These guidelines suggest that e-cigarettes may aid in smoking cessation (IIb, C); however, it is advisable to limit their use and avoid simultaneous use with conventional cigarettes due to unknown long-term effects. Given that their use has been clearly associated with adverse effects on CV, respiratory, immunological, and periodontal health compared with nonuse, we find this controversial guideline recommendation to be striking.
FUNDINGThis article has not received any funding.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
SEC Guidelines Committee: Pablo Avanzas (president), Pilar Mazón, (secretary), Rut Andrea Ribas, Marisol Bravo Amaro, Alberto Cordero Fort, Marisa Crespo, F. Javier Jiménez Candil, María Antonia Martínez Momblan, Sonia Mirabet Pérez, Juan Sanchis Forés, Marta Sitges Carreño, José M. de la Torre, Javier Torres Llergo, and David Vivas.
SEC Working Group for the 2024 ESC guidelines for the management of peripheral arterial and aortic diseases: Javier Torres Llergo (coordinator), Raquel Campuzano Ruiz (coordinator), José Antonio Alarcón Duque, Juan José Gómez Doblas, Rafael Mesa Rico, Soledad Ojeda Pineda, Patricia Palau Sampio, and Enrique María San Norberto García.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.10.002
See related article: https://secardiologia.es/cientifico/guias-clinicas/prevencion-riesgo-cardiovascular/15238-2024-esc-guidelines-for-the-management-of-peripheral-arterial-and-aortic-diseases
The names of all the authors of the article are listed in alphabetical order in Appendix A.
Corresponding author. E-mail address: javiertorresllergo@gmail.com (J. Torres Llergo).