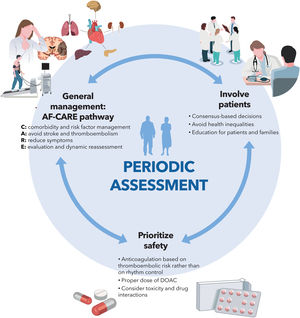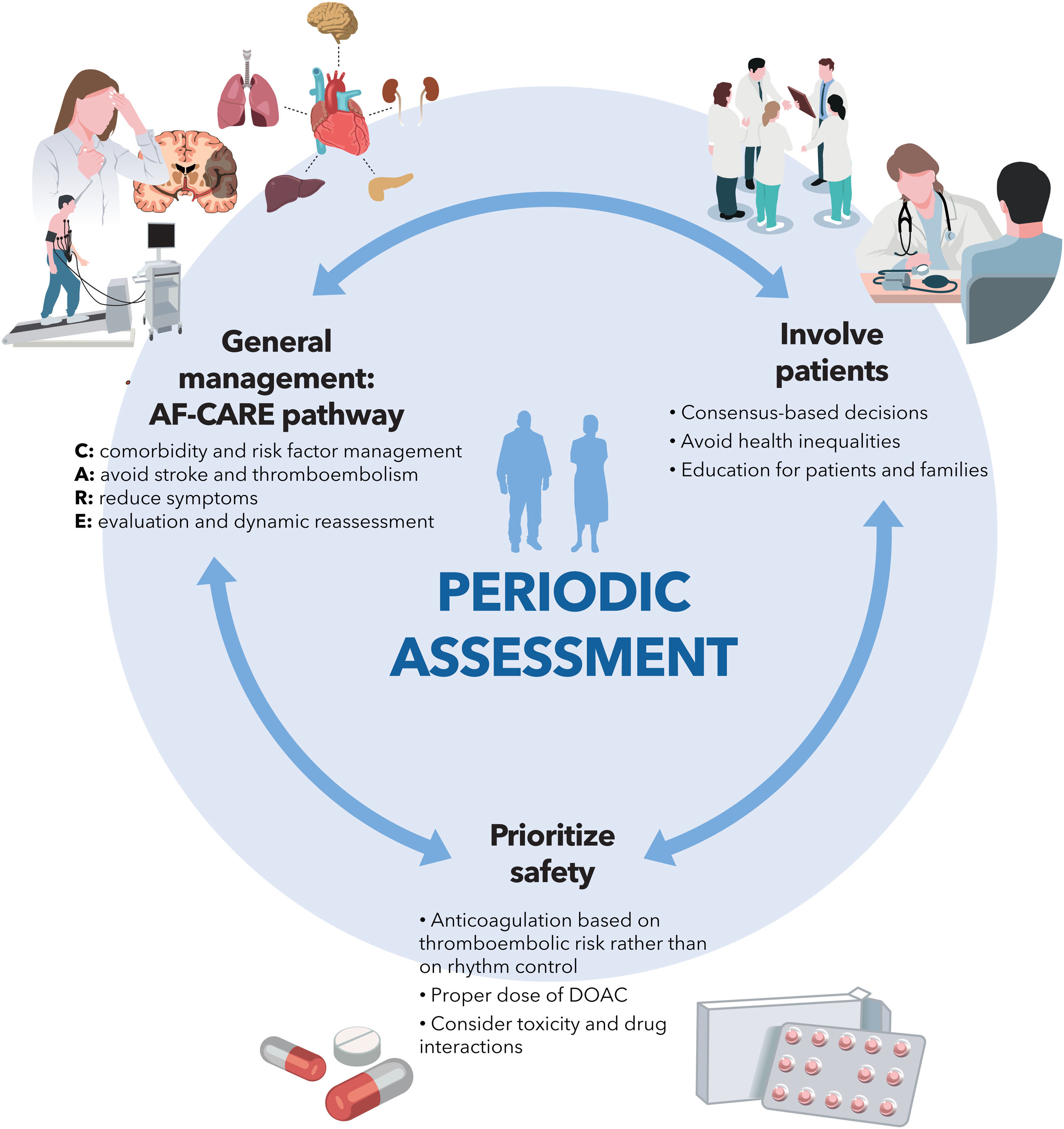
The new version of the European Society of Cardiology clinical practice guidelines on atrial fibrillation (AF) provides a comprehensive review of the state of the art in the diagnosis, prevention, and treatment of AF. The most relevant messages (figure 1) include a new general management scheme, called AF-CARE, which is notable for its comprehensive and multidisciplinary approach (focused on the detection and treatment of risk factors), prioritizing the reduction in symptoms and the prevention of thromboembolism, within a clinical pathway that includes periodic re-evaluation. In addition, the guidelines emphasize the need to involve patients—and society as a whole—in the management of AF, avoiding inequities due to gender, race, or social status. Safety remains a priority, requiring careful use of therapeutic tools that must be evaluated periodically to avoid undesirable effects. Below is a detailed and critical commentary on the novel features, limitations, and implications of these guidelines.
WHAT IS NEW IN THE 2024 GUIDELINESDefinitions and clinical impact“Clinical AF” refers to electrocardiogram (ECG)-diagnosed AF, whether symptomatic or asymptomatic. In contrast, “device-detected subclinical AF” includes asymptomatic episodes detected by implantable or portable devices and subsequently confirmed by a competent professional. The term “early AF” is now included, referring to AF with onset between 3 and 24 months previously. “Atrial cardiomyopathy” is given less emphasis than in previous guidelines. As expected, an echocardiogram is recommended in all patients with a new diagnosis of AF to guide therapeutic decisions beyond anticoagulation.
AF-CARE clinical pathwayThe AF Better Care (ABC) approach in previous guidelines has been replaced by the AF-CARE pathway, which promotes patient-focused multidisciplinary collaboration organized around 4 pillars: management of comorbidities and risk factors (C), avoidance of stroke and thromboembolism (A), symptom relief through rate or rhythm control strategies (R), and dynamic assessment over time (E).
The guidelines highlight the importance of improving the patient's metabolic profile to reduce the AF burden and maximize the effectiveness of interventions such as catheter ablation1 through a cross-sectional and multidisciplinary approach. Furthermore, emphasis is placed on the patient being an active participant in treatment and decision-making to achieve optimal health outcomes and avoid inequities (figure 1).
C: comorbidity and risk factor managementThe inclusion of this concept is not new, as it is explicitly mentioned in other documents on AF. However, an important point is that AF-CARE places the management of comorbidities and risk factors as the initial component of patient care. This approach is based on the assumption that it should be considered first and applies to all AF patients. Since comorbidities and risk factors are related to the pathogenesis of AF, the management of AF is more effective when they are properly addressed.2 This includes, but is not limited to, managing hypertension, heart failure, diabetes, obesity, sleep apnea, physical activity and alcohol intake through appropriate pharmacological and nonpharmacological interventions. All these aspects receive a high class of recommendation and level of evidence in these guidelines. In addition, other comorbidities may require special attention, such as the management of dyslipidemia and vascular disease, smoking cessation, and even exposure to air pollution. It is also recognized that achieving effective management of comorbidities requires several lifestyle changes. This involves a complete behavioral shift to adhere to a healthy lifestyle; therefore, shared decision-making and a multidisciplinary approach with the support of primary care physicians, cardiologists, internists, and nurses are essential.
A: avoid stroke and thromboembolismDue to the disparities in the application of the usual thromboembolic risk scores in real-world patients, the guidelines initially refrain from endorsing any particular score. Instead, oral anticoagulation (OAC) is recommended in patients at high thromboembolic risk to prevent stroke (class I-A), following an individualized assessment of all risk factors. Although no thromboembolism risk score is allocated for universal use, the guidelines recommend both the application of locally validated scales and the CHA2DS2VA score (in which the female sex component has been removed since it functions as an age-dependent stroke risk modifier rather than a risk factor).
OAC is also recommended in patients with AF and hypertrophic cardiomyopathy or cardiac amyloidosis (I-B), regardless of their CHA2DS2VA score.
Emphasis is placed on the need for appropriate dosing of direct-acting oral anticoagulants (DOAC), avoiding unjustified dose reductions, as this practice increases the risk of stroke without decreasing the risk of bleeding. The results of the FRAIL-AF trial3 are highlighted, showing that in a population of AF patients aged ≥ 75 years with frailty, polypharmacy, and a stable international normalized ratio with good time in therapeutic range, switching from vitamin K antagonists (VKA) to DOAC was associated with an increase in bleeding. Therefore, switching from VKA to DOAC in these patients carries a Class IIb-B recommendation.
Since up to one-third of patients with AF on OAC experience an ischemic stroke, it is recommended to assess noncardioembolic causes of stroke, evaluate risk factors, ensure adherence, and determine the appropriate dose of anticoagulants (IIa-B).
The assessment of bleeding risk using a scoring system has been replaced by an individualized evaluation of bleeding risk factors (I-B). Together with idarucizumab, adexanet *alfa has also been incorporated into the management of life-threatening or critical organ bleeding (IIa-B).
Following the publication of the results of the NOAH and ARTESIA trials, the ESC guidelines recommend considering anticoagulation in certain subgroups of device-detected subclinical AF patients, particularly those with a high thromboembolic risk and no major bleeding risk factors (IIb-B). A recent meta-analysis of the NOAH and ARTESIA trials4 examined outcomes based on the presence or absence of vascular disease, showing a 34% reduction in the risk of stroke and systemic embolism in the anticoagulation group.
The guidelines retain the indication for percutaneous left atrial appendage occlusion (LAAO) in patients with contraindications for OAC (IIb-C). However, uncertainty persists regarding the optimal antithrombotic treatment postimplantation, given that DOAC carry a similar bleeding risk to aspirin. Regarding surgical LAAO, there are 2 new indications: a) concomitant with endoscopic or hybrid AF ablation as adjunctive therapy with anticoagulation (IIa-C), and b) isolated endoscopic LAA closure in patients where anticoagulation is contraindicated (IIb-C).
R: symptom reduction through rate and rhythm controlTwo new recommendations have been established for rate control. Digoxin is now considered as a first-choice drug, along with beta-blockers and calcium antagonists, in patients with AF and left ventricular ejection fraction (LVEF)> 40%, for rate control and symptom reduction (I-B). Atrioventricular (AV) node ablation combined with cardiac resynchronization therapy is now the first treatment option in patients with severe symptoms, permanent AF, and at least 1 hospitalization for heart failure.
A rhythm control strategy should be implemented within 12 months of diagnosis in selected patients with AF, risk of thromboembolic events, and/or other conditions (chronic kidney disease, left ventricular hypertrophy), to reduce the risk of cardiovascular death and hospitalizations, according to the published evidence.5 However, it should be emphasized that the rhythm control strategy does not determine the indication for OAC, which should be carried out according to the risk factors for thromboembolism (figure 1).
Catheter ablation is now considered the first-line therapeutic option in patients with paroxysmal AF. Other possible indications include its use in patients with AF-related bradycardia or pauses after AF termination. Repeating the procedure is recommended in cases of recurrence if the patient experienced symptom improvement or if the initial procedure was ineffective. The indications for ablation remain unchanged in patients with AF and heart failure with reduced LVEF, or persistent AF. AF ablation procedures should be performed without interrupting OAC.
Cardioversion (whether pharmacological or electrical) is advised in symptomatic patients with persistent AF as part of an initial rhythm control strategy. Electrical cardioversion can also be used as a diagnostic tool in patients with persistent AF when there is uncertainty about possible improvements in symptoms or ventricular function. DOAC should be used in preference to VKA. Cardioversion should not be performed in episodes lasting ≥ 24hours without prior adequate anticoagulation, or if a transesophageal echocardiogram cannot be performed.
In the surgical field, endoscopic or hybrid procedures are recommended in patients with persistent symptomatic AF who are refractory to drug therapy (IIa-A). Among patients with symptomatic paroxysmal AF and failure of percutaneous ablation, the indication for surgical ablation has been downgraded to Class IIb-B. Surgical ablation is advised in patients who are candidates for a rhythm control strategy and are undergoing cardiac surgery, regardless of whether it involves the mitral valve.
E: evaluation and dynamic reassessmentThis is the first time that the need for dynamic risk assessment has been explicitly included in a specific care pathway. This is a key point, as the risk in AF patients is not static; rather it is dynamic and changes over time.6 Therefore, periodic reassessment throughout the disease continuum is required. In this process, several issues should be considered and reviewed, including medication changes and optimization, clinical and imaging re-evaluation, and risk factor management.
The AF-CARE pathway in specific clinical contextsThe new guidelines describe new clinical scenarios not mentioned in the previous guidelines, such as AF in unstable patients, AF precipitated by a trigger, and AF in cancer patients, with a brief mention of atrial flutter. However, other relevant clinical contexts are not discussed, such as heart failure, valvular heart disease, gastrointestinal and hematological diseases, and chronic kidney disease. Among AF patients with coronary artery disease, the recommendation, already outlined previously, to minimize the duration of triple therapy by stopping aspirin 1 week after stent implantation is reiterated, except in patients with a high thrombotic risk, in whom triple therapy can be maintained for 1 month (and up to 3 months in patients with diabetes and very high thrombotic risk). DOAC are also prioritized over VKA in this context, and it is strongly recommended not to extend antiplatelet therapy beyond 12 months in patients with stable coronary or peripheral artery disease treated with OAC.
In patients with AF triggered by an external factor, long-term OAC is advised if the thromboembolic risk is high, as both AF recurrences and thromboembolism are frequent.
Regarding the prevention of postoperative AF, amiodarone is prioritized over beta-blockers when preventive pharmacological treatment is desired; however, precise indications are not detailed. For the first time, the guidelines recommend the performance of posterior pericardiotomy during cardiac surgery to prevent postoperative AF (IIa-B).7 The indication for long-term OAC is maintained if the thromboembolic risk is high.
In patients with embolic stroke of unknown source (ESUS), it is recommended to prolong cardiac rhythm monitoring based on the presence of AF risk markers. In addition, it is explicitly discouraged to initiate OAC in the absence of documented AF, given its lack of efficacy.
Screening and prevention of atrial fibrillationAs in 2020, a specific section is devoted to detailing the various diagnostic tools and portable devices, although their characteristics are better specified by classifying them according to a fundamental criterion: whether they are based on ECG acquisition or other methods, such as pulse palpation, oscillometry, photoplethysmography, mechanocardiography, or smart speakers. As a key point, the guidelines acknowledge the low quality of the available evidence on smartphone apps based on photoplethysmography, whose sensitivity and specificity values have probably been overestimated.8 Consequently, the recommendation from previous guidelines is downgraded. Thus, non-ECG-based methods are considered as adjuvant in AF screening, but never diagnostic per se.
The potential usefulness of single opportunistic screening in at-risk populations (≥ 65 years) is mentioned, but is assigned a grade C recommendation and is always contingent upon obtaining an ECG. As in 2020, systematic population screening is recommended (IIa-B) for individuals aged ≥ 75 years or ≥ 65 years with “additional CHA2DS2VA risk factors”.
The document also explores the potential usefulness of predictive algorithms for AF occurrence based on machine learning techniques, while also considering the current limited value of genetic screening.
Finally, a much more extensive section is dedicated to the primary prevention of AF, discussing arterial hypertension, heart failure, type 2 diabetes mellitus, obesity, sleep apnea syndrome, physical activity and alcohol consumption in greater detail.
LIMITATIONS AND GAPS IN EVIDENCEDefinitions and clinical impactThe diagnosis of AF requires confirmation by a conventional ECG or by recording 1 or more leads for at least 30seconds: this duration cut-off, although derived from expert consensus, is arbitrary.
The classification of AF according to the temporal pattern—into first-diagnosed, paroxysmal, persistent, and permanent (for patients in whom a rhythm control strategy is considered futile)—remains unchanged from previous guidelines. The rationale for retaining this terminology is that the evidence available for therapeutic decision-making stems from clinical trials that have used these definitions, despite their failure to reflect the underlying pathological process.
The time interval defining “early AF” is too wide (3-24 months) and difficult to translate into clinical practice. While recognizing the importance of reducing the “AF burden”, which affects stroke risk and quality of life, we lack clarity on the extent of reduction necessary to achieve meaningful clinical impact.
A: avoid stroke and thromboembolismReplacing population-based scores with multivariate models that include biomarkers and structural factors could improve the estimation of the risk of thromboembolism, although this remains unproven. Identifying patients with AF who are truly at low risk of thromboembolism is a persistent challenge, regardless of the predictive model used.
OAC should probably be extended to include other restrictive cardiomyopathies beyond hypertrophic cardiomyopathy and amyloidosis, as noted in previous guidelines.
Furthermore, more precise data are needed to identify patients who would derive the greatest benefit from the use of DOAC in device-detected subclinical AF.
R: symptom reduction through rate and rhythm controlThe role of conduction system pacing combined with AV node ablation is mentioned, but no specific recommendation is provided. Nonetheless, this approach is used more frequently in routine clinical practice than cardiac resynchronization.9
Among patients with AF lasting more than 12 months, the prognostic implications of a rhythm control strategy, as well as the roles of AV node ablation and conduction system pacing, require further investigation.
The guidelines still lack criteria based on cost-effectiveness studies on catheter ablation.10 There are also numerous gaps in evidence, according to the 9th AFNET/EHRA consensus conference.11 Addressing these issues could help reduce the imbalance between the number of patients potentially eligible for some of these techniques and the limited available resources.
E: evaluation and dynamic reassessmentThe most appropriate timing for reassessment of patients with AF remains unclear, although the guidelines recommend re-evaluation 6 months after the baseline presentation and at least annually thereafter. Nevertheless, follow-up and regular reviews should be individualized and adapted depending on each patient's clinical status.
The AF-CARE pathway in specific clinical contextsThe combination of AF and chronic kidney disease is an important clinical situation due to its high prevalence and the difficulties it poses for OAC. However, there are no specific recommendations on this topic.
Equally, the guidelines do not discuss whether AF occurring in the acute phase of myocardial infarction requires a special approach, likely due to limited evidence. In this context, OAC increases bleeding risk more than in other settings because it must be combined with antiplatelet therapy. OAC also significantly impacts the selection and duration of antiplatelet therapy. Therefore, a specific recommendation could be warranted.
Finally, the optimal duration of continuous ECG monitoring after ESUS remains unknown.
Screening and preventionWhile the recommendation for single opportunistic screening is maintained, there is no evidence from clinical trials that it increases the detection of AF12 or that it reduces clinical events.
The recommendation for systematic population screening seems too vague, as it remains unclear which patient groups would derive the greatest benefit, the optimal screening duration, and the burden of AF detected that should be considered “at risk”.
CLINICAL IMPLICATIONSA multidisciplinary approach to patients with AF necessitates the implementation of specific, holistic and comprehensive clinical pathways that extend beyond the field of cardiology and involve health care professionals from primary care, geriatrics, internal medicine, endocrinology, pneumology, and nursing, as well as patients and families. A key element of this clinical pathway is periodic re-evaluation of patients,13 particularly to monitor disease progression, comorbidities, thromboembolic risk, and medication (ie, DOAC dosage).
FUNDINGThis article has not received any funding.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
CONFLICTS OF INTERESTThe conflict-of-interest declaration documents for all authors are available in the supplementary data.
SEC Guidelines Committee: Pablo Avanzas (president), Pilar Mazón (secretary), Rut Andrea Ribas, Marisol Bravo Amaro, Alberto Cordero Fort, Marisa Crespo, Javier Jiménez-Candil, María Antonia Martínez Momblan, Sonia Mirabet Pérez, Juan Sanchis Forés, Marta Sitges Carreño, José M. de la Torre, Javier Torres Llergo, and David Vivas.
SEC Working Group for the 2024 ESC Guidelines for the management of atrial fibrillation: Javier Jiménez-Candil (coordinator), Concepción Alonso (coordinator), José A. Barrabés, Olga Duran-Bobin, Javier García-Seara, Beatriz Jáuregui, Rafael Peinado, and José Miguel Rivera-Caravaca.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.10.011
Corresponding author. E-mail address:jimenezcandil@secardiologia.es (J. Jiménez-Candil).
SEE RELATED CONTENT:https://secardiologia.es/cientifico/guias-clinicas/arritmias/15235-2024-esc-guidelines-for-the-management-of-atrial-fibrillation.