Keywords
INTRODUCTION
Percutaneous coronary intervention (PCI) is the treatment of choice in saphenous vein stenosis (SVS) due to the increased mortality associated with repeat revascularization surgery.1 However, although the appearance of bare metal stents (BMS) improved results of balloon angioplasty in SVS, stent restenosis remains the major limitation of PCI in this context.2
Drug-eluting stents (DES) have brought about a substantial reduction of the restenosis rate in native vessels.3 Their use in SVS is controversial because, although previous studies have been published, data on survival and repeat revascularization vary greatly. To date, existing studies are limited for various reasons: non-randomized design,4-13 retrospective nature, absence of control group,4,8,12,14 limited number of patients4,5,9,11,13,14, or short follow-up period.7,8,10,11 It is imperative we determine the benefits of these devices in SVS because, although in Spain they represent just 2.6% of all stenoses treated,15 in other countries they account for up to 10%-15%.16 Moreover, DESs are used in a very high percentage of these stenoses even though the recent guidelines indication is IIb.17
In the present paper, we study the immediate and long-term evolution of a series of consecutive patients treated with DES in de novo SVS and compare this with the evolution of a consecutive series of patients also with de novo SVS treated with BMS. Our primary objectives are to study the long-term evolution in terms of cardiac death and target vessel revascularization and to determine predictors of cardiac death and repeat revascularization.
METHODS
Patients
From the intervention registers of the 5 participating hospitals, we selected all patients undergoing PCI with DES in at least 1 SVS from initial approval of DES in each center to July 1, 2007. During this period, in all 5 hospitals DES were the only stents deployed in SVS unless patients were contraindicated for prolonged double antiplatelet therapy. Data on this cohort was compared with that of a historic series of patients with BMS in saphenous vein graft from 2 of the participating centers.18 Demographic and procedural characteristics were obtained from these registries where they had been introduced prospectively. Follow-up data was obtained retrospectively. Quantitative analysis was conducted off-line with the previously-validated edge-detection systems existing in each center (CAAS II, V4.1.1. Pie Medical Imaging Maastricht, The Netherlands; Medis Medical Imaging Systems Inc, Leiden, Netherlands). To study the possible influence of DES in cardiac death, all patients were analyzed in a single group using regression techniques; lesions were treated similarly for final repeat target vessel revascularization.
Procedure
Indications for revascularization included all forms of coronary heart disease and primary angioplasty. There were no restrictions on type of stent, guide catheter or on use of glycoprotein IIb/IIIa inhibitors. Choice of material, mode of use and drug regimen were at the discretion of the interventional cardiologist responsible. Following center protocols, intravenous heparin was administered to achieve adequate activated coagulation time. Decisions on using distal filters or performing dilatation following stenting were also left to individual operators.
All patients underwent pre- and postintervention ECGs to detect new ischemic events and CPK, CPKMB and/or troponin T were determined at 8 and 24 hours, following center protocols.
Definitions
Procedure success: <20% residual stenosis and TIMI flow 3 in the obstructed vessel without further complications. Thrombus: contrast-surrounded filling defect opacification visible in multiple projections. Myocardial infarction: twice normal CPK-MB elevation or 10 times normal troponin T elevation.
Follow-up
Follow-up was by telephone. Information on patients who died was obtained from family, referring physicians and clinical records to determine cause of death and events occurring prior to death. No center conducted systematic angiography at follow-up; this was only used when indicated by clinical criteria.
Statistical Analysis
Continuous variables are expressed as mean (SD) and categorical variables as absolute values and percentages. We used Student t test to compare means, c2 to compare proportions in normally distributed variables, and non-parametric test for asymmetric distributions. All studies used 2 tail analysis and P≤.05 was considered significant. We calculated Kaplan-Meier curves for survival and repeat target vessel revascularization. Cox regression models included all patients in a single cohort with the baseline clinical and procedural characteristics the authors considered might associate with poor prognosis to determine predictors of long-term cardiac death. We also used Cox regression for all lesions for repeat target vessel revascularization. In multivariate analysis, all quantitative variables, including ejection fraction, were treated as continuous. Data were analyzed with SPSS 15.
RESULTS
We found significant differences in baseline characteristics of older DES patients and in trends towards more women, patients with diabetes, previous PCI, and anti-GP IIb/IIIa therapy in the BMS group, and towards older grafts in the DES group. Table 1 summarizes patients' clinical characteristics.
Table 2 shows characteristics of the stenoses. Except for a greater percentage of thrombus present in the BMS group, we found no differences in vessel type, location, reference diameter, stenosis length, preintervention minimal lumen diameter, or severity. We also include results for stenosis. Despite similar pre- and postintervention reference diameters, smaller caliber and longer stents were chosen in DES patients. In DES patients, direct stenting was less common, and filters and post-dilatation tended to be more frequent.
Table 3 shows intrahospital clinical results. Event frequency of was very low with no differences except a trend towards more frequent periprocedural infarction in BMS patients.
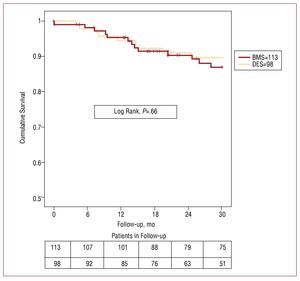
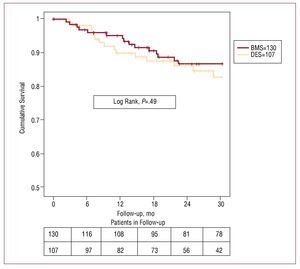
Clinical follow-up was 99% in BMS and 98% in DES patients. We found no significant differences in absence of cardiac death or target stenosis revascularization rate. Absence of cardiac death at 12, 24, and 30 months was: DES, 95% (2%), 91% (3%), and 89% (3%), respectively; and in BMS, 95% (2%), 90% (3%), and 87% (3%), respectively (P=.66). Absence of target vessel revascularization at 12, 24, and 30 months was: 90% (3%), 86% (4%), and 83% (4%) in DES and 94% (2%), 87% (3%), and 87% (3%) in BMS (P=.49). Figures 1 and 2 show survival curves for both variables. Of 9 DES patients who died of cardiac or unknown causes, we found none had definitive or probable thrombosis as defined by Academic Research Consortium criteria. These same criteria indicate that 2 patients who died of unexplained causes at 4 and 5 months and should be considered possible cases of stent thrombosis.
Figure 1. Without cardiac death. The upper row gives figures on the number of patients in the bare metal stent (BMS) group and the lower row gives figures on the drug-eluting stent (DES) group, at 6 month intervals.
Figure 2. Without repeat revascularization. The upper row gives figures on the number of lesions in the bare metal stent (BMS) group and the lower row gives figures on the drug-eluting stent (DES) group, at 6 month intervals.
Finally, we constructed Cox regression models for the variables DES, diabetes mellitus, ejection fraction, creatinine, age, presence of thrombus, length of stent, anti-GPIIb/IIIa, and direct stenting. Only ejection fraction behaved as an independent predictor of cardiac death although kidney function showed a trend towards predicting this outcome.
With the same procedure, we analyzed repeat target vessel revascularization with variables DES, diabetes mellitus, creatinine, presence of thrombus, stent length, and stent caliber. None proved statistically significant. Table 4 shows multivariate analysis data for cardiac death and target vessel revascularization.
DISCUSSION
In the present study, we compare results of DESs and BMSs in saphenous vein graft stenosis. Our most important findings are that DES use does not associate with a difference in cardiac death or a reduction in the repeat target vessel revascularization rate. Ejection fraction was the only predictor of cardiac death and we found no predictor of repeat revascularization. Finally, although it lay outside the objectives of our study, we found the use of filters was very low.
Intrahospital Results
In our series, we found no differences in mortality or repeat revascularization, with almost nonexistent intrahospital rates for both events. These results coincide with all series that compare DES and BMS stents.5-7,19
We found a higher percentage of myocardial infarction tended to associate with stenting in the BMS group. This was not statistically significant (P=.13) and may have been due to the small sample size. Most such events were minor non-new Q-wave infarctions. The benefits of direct stenting over predilatation on the myocardial infarction rate in SVS have been demonstrated18 and in our series direct stenting was more frequent in BMS, probably due to the shorter stent length in this group. However, this difference has several possible explanations: a) preintervention presence of thrombus was more frequent in the BMS group, perhaps because double antiplatelet therapy was less common at the time when BMSs were deployed than in the period when DESs were (this coincides with the greater use of abciximab in the BMS group, possibly because of the not infrequent presence of thrombus in these patients and because when BMS stenting was common, the failings of abciximab in SVS had not been demonstrated20); b) as reported in previous studies, in the BMS group, comparatively larger diameter stents were deployed in similar caliber vessels so as to increase final lumen diameter; in DES patients this is less aggressive as operators trust in the efficacy of the drug; furthermore, especially in the initial stages of the study, the availability of large caliber stents in the DES group was limited and this may have contributed to the difference; here, our results coincide with Chu et al5 who also found a lower infarction rate and suggested smaller diameter DESs may be responsible; and c) PCI guidelines recommend using distal protection devices in SVS,17,21 and DES group patients received them more often because of their increasing availability over time. Notwithstanding, very few distal protection devices were deployed, probably, for two reasons. Firstly, in Spain they are used far less than is recommended. In 2006, only 235 devices were delivered in 1472 saphenous vein procedures, representing 16% of the total and a 10% fall over 2005.15 Secondly, one of the participating centers that enrolled most of the patients, has not approved use of these devices.
Long-Term Survival
We found no differences between the 2 groups in terms of cardiac death. Although the study is not randomized and, consequently, from the outset we had a significant difference with older patients in the DES group and more frequent presence of thrombus in the BMS group, together with a tendency towards more patients with diabetes and younger grafts in the BMS group, when we analyzed factors predicting cardiac death only ejection fraction achieved statistical significance, with DES having no influence. To avoid any crossover of patients from one group to the other and the possible associated bias, our study only included de novo stenosis and excluded patients stented for restenosis.
In the literature, results for long-term survival vary (Table 5). Most studies are limited by short follow-up and to date, only 2 series have >1 year follow-up. At present, the RRISC trial14 is the only randomized study, with 35 patients in the DES group and 37 in the BMS group, excluding patients presenting myocardial infarction in the 7 days preintervention, those with <25% ejection fraction, creatinine >3 mg/dL or distal anastomosis stenosis. After nearly 3 years follow-up, a significant increase in mortality in the DES group was found, with 11 deaths versus 0 in the BMS group. Currently, this is the only study to find increased mortality during DES group follow-up. The RRISC trial has the most appropriate design as it is randomized. However, the limited number of patients, making evaluation of clinical events inappropriate, and the fact that 4 of the 11 deaths were of noncardiac origin, mean the results, although very important, should be interpreted with care.22 In contrast, 3 of the 7 cardiac deaths were from sudden death, and in this study, although at the end of the follow-up half the patients were receiving double antiplatelet therapy, the obligatory recommendation was for 2 months treatment only. Consequently, some of these events may be related with stent thrombosis since patients were not taking adequate antiplatelet therapy. The second study with nearly 3 years follow-up is by Bansal et al,4 who found no differences between groups. Studies with shorter follow-up periods also show mixed results as some find no differences,5-8 whereas Lee et al10 do report benefits from the use of DESs. However, these benefits (1 death in the BMS group and 4 in the DES group; P=.03) were found after a mean follow-up of only 9 months and the authors themselves query whether the benefits would be maintained during a longer follow-up. Finally, the DES series with no control group, all of which include <12 months follow-up, present satisfactory results with few deaths.9,11-13
Repeat Revascularization
We found no differences between groups in the repeat target vessel revascularization rate, either. As we comment above, our study only included de novo stenosis to avoid the crossover of patients from one group to the other and any associated bias. Results in series published elsewhere also vary (Table 5) as some found benefits7,8,10,19 but others reported similar results.4-6 Although stenosis in saphenous veins behaves differently to that in native vessels because the disease progresses more diffusely with a later, more aggressive pattern of coronary heart disease development,2,23 like in native vessels, we can be sure that DESs present less late loss than BMSs as all saphenous vein studies with protocol angiographic follow-up have shown significant reduction of late loss and restenosis rate. The only randomized study, RISCC, found a very significant reduction of late loss (0.71 [0.61] vs 0.4 [0.51] mm; P=.015) in the DES group at 6-month follow-up, although this benefit would finally disappear after 3 years.14 With angiographic follow-up approaching 70% in both groups, Ge et al7 report a significantly greater target vessel revascularization rate in BMS (4.9% vs 23.1%; P=.003). Finally, with 80% follow-up angiography, Hoffmann et al8 report a 6% target stenosis revascularization rate versus 22% (P=.024). However, what remains less clear is whether the rate of revascularization on clinical indication falls with DES. From previous studies, we know that angiographic restenosis is approximately twice clinical restenosis24 and that follow-up angiography leads to notable increases in the repeat revascularization rate. The only study which shows a benefit from DES without protocol follow-up angiography was by Lee et al10 who found target vessel revascularization of 10% versus 37% (P=.035). Lee et al reported follow-up angiography was conducted in 30.2% of DES and 66.7% of BMS and, although it was performed on theoretically clinical criteria, percentage angiographic follow-up is much higher than in other studies with angiographic follow-up for clinical criteria. These studies failed to demonstrate the superiority of DES.4-6 None of our participating centers conducted follow-up angiography, which means our results coincide with those of existing studies.
Predictors of Cardiac Death
Previous studies comparing DES with BMS in saphenous vein graft stenosis found predictors of mortality such as diabetes mellitus, BMS, graft age, stent length and presence of thrombus.7,12 Ejection fraction has been proven an independent predictor of mortality in many previous studies.18,25-27 In our series only ejection fraction was a statistically significant independent predictor of cardiac death although kidney failure showed a tendency to predict death. In a larger sample of patients, it may have been shown to be an independent predictor, too.
Limitations
The study compares 2 cohorts of patients, one enrolled from interventions performed between 1999 and 2002 in 2 centers and the other of patients undergoing procedures with DES in 5 centers between 2003 and 2007. Consequently, the study design may have introduced differences related to the range of centers; similarly, the fact the control group is drawn from different periods may have introduced a bias.
Furthermore, we found differences in patients' baseline characteristics due to the lack of randomization, with older patients and less frequent presence of thrombus in the DES group, and a trend towards higher percentages of patients with diabetes, of women, and lower graft age in the BMS group. Furthermore, in both groups different models of stents were used and we assume they all behaved similarly in each group. We cannot discount a bias derived from treating all stents in this way.
Follow-up applied the centers' standard procedures and did not include testing to detect ischemia or systematic angiography. We cannot discount the chance that revascularization rates might have differed had these studies been conducted.
CONCLUSIONS
The use of DES in saphenous vein graft stenosis does not associate with differences in cardiac death by comparison with BMS. In our series, we found no association with a difference in repeat target vessel revascularization rate. Ejection fraction was the only independent predictor of cardiac death and we found no predictors of repeat revascularization. Finally, one finding that arose incidentally and was not included among the objectives of the study was the fact that the rate of filter use was very low.
ABBREVIATIONS
BMS: bare metal stent
DES: drug-eluting stent
PCI: percutaneous coronary intervention
SVS: saphenous vein stenosis
Correspondence: Dr. I. Lozano.
2132 Carretera Piles-Infanzón.
33203 Gijón.Asturias. España.
E-mail: imlml9@hotmail.com
Received February 21, 2008.
Accepted for publication August 29, 2008.