The SAPIEN 3 (S3) valve and the Medtronic Evolut R (EVR) are second-generation transcatheter valves, designed to further reduce the rate of paravalvular aortic regurgitation (AoR). The aim of this study was to compare the 2 devices in terms of valve performance in a case-matched study with independent echocardiographic analysis.
MethodsOf a population of 201 patients who underwent transcatheter aortic valve implantation, 144 patients (S3, n = 80; EVR, n = 64) were matched according to aortic annulus diameter and aortic valve calcium score, as assessed by computed tomography. All echocardiographic examinations collected at baseline and at 1- and 6-month follow-up were centrally analyzed.
ResultsThe 2 groups were well balanced in baseline clinical and echocardiographic characteristics. The EVR valve showed a better hemodynamic profile as assessed by peak aortic gradient (EVR 13 ± 7 vs S3 20 ± 10mmHg; P<.001), mean aortic gradient (EVR 7 ± 3 vs S3 11 ± 6mmHg; P<.001), and Doppler velocity index (EVR 0.65±0.15 vs S3 0.51±0.16; P<.001). The rate of moderate-severe or any paravalvular (≥ mild) AoR was higher in the EVR group (11% and 50%) than in the S3 group (2.5% and 21%; P <.05, respectively), with a larger number of paravalvular jets (P <.001).
ConclusionsIn a case-matched cohort of transcatheter aortic valve implantation patients, the S3 valve was associated with a lower rate of paravalvular AoR but also with a higher residual gradient than the EVR system.
Keywords
Transcatheter aortic valve implantation (TAVI) is recommended in patients with severe aortic stenosis considered inoperable or at high surgical risk and is an alternative to surgery in intermediate risk patients.1 The presence of paravalvular leakage resulting in moderate-severe aortic regurgitation (AoR) is one of the major limitations of early generation valves because of its association with increased late mortality2–4 and higher incidence compared with surgical valves.5,6 The new-generation valves, the balloon-expandable Edwards SAPIEN 3 (S3) (Edwards Lifesciences, Irvine, California, United States) and the self-expandable Medtronic CoreValve Evolut R (EVR) (Medtronic, Minneapolis, Minnesota, United States) have been modified from its predecessors to further reduce the rate and severity of paravalvular leakage, increase device success and lower the rate of procedure-related complications. The S3 incorporates an external sealing cuff at the bottom of the stent frame to reduce the incidence of paravalvular AoR7; while the EVR incorporates a sealing skirt extended at the in-flow aspect of the valve, and a repositionable system developed to optimize valve positioning.8,9 However, direct comparison between these 2 newer generation valves is lacking. The objective of this study was, therefore, to compare the hemodynamic performance of patients undergoing TAVI with S3 vs EVR valves in a case-matched population, as assessed by an independent analysis.
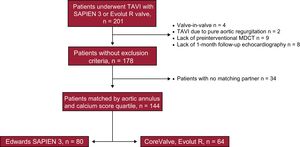
METHODSA total of 201 consecutive symptomatic patients who underwent TAVI from 2 centers were eligible. Suitability and eligibility for TAVI were determined by the Heart Team at each institution. Both centers had experience with these 2 new-generation transcatheter valves and included patients with both S3 and EVR. Patients with previous mitral or aortic valve, high risk of coronary occlusion, or annulus rupture were preferably treated with EVR and patients with intraventricular conduction abnormalities (especially right bundle branch block) and very tortuous or horizontal aorta were preferably treated with S3. Patients with pure AoR, previous aortic valve replacement (valve-in-valve procedures), lack of a preinterventional echocardiogram or cardiac computed tomography, or absence of 30-day postinterventional echocardiogram were excluded. Informed consent was obtained in each patient before the procedure. Eligible patients were matched according to: a) aortic annulus diameter derived by perimeter (within 0.5mm of difference), and b) aortic valve calcium score quartile as assessed by multidetector computed tomography (MDCT). A variable number of controls (from 1 to 3) were allowed, leading to a final sample of 144 patients (S3=80 and EVR=64) (Figure 1). Multidetector computed tomography was performed with a 64-row detectors electrocardiogram-gated helical scan during the infusion of nonionic iodine contrast. Retrospective acquisition with 0.625mm slices and 50% overlap were used per TAVI planning. Multidetector computed tomography examinations were performed and interpreted in accordance with Society of Cardiovascular Computed Tomography guidelines10 using the IntelliSpace V. 4.0 software (Philips Medical Systems, Andover, Massachusetts, United States). As part of the protocol study, the following derived parameters were calculated: aortic annulus (minimum, maximum diameters, perimeter, and area), perimeter- and area-derived diameters, and distance from the aortic annulus to the coronary arteries. Aortic valve calcification was graded quantitatively by the aortic valve calcium score as previously described.11 Briefly, a region of interest for calcium quantification was made from the basal annular plane to the leaflet tips excluding coronary calcium, with a threshold for calcium detection set at 130 Hounsfield Units. Prosthesis sizing was determined on the basis of aortic annulus measurements using the manufacturer's guidelines for sizing (). The objective was to obtain a 1% to 15% prosthesis area oversizing with respect to the aortic annulus area in all patients. The eccentricity index [defined by 100 × [1-(aortic annulus minimum diameter/maximum diameter)], sizing index (defined by nominal transcatheter valve diameter or area/aortic annulus diameter or area), and cover index [defined by 100 × (nominal transcatheter valve diameter – MDCT diameter)/nominal transcatheter valve diameter] were also calculated.
All the included patients underwent a complete transthoracic echocardiographic examination according to the guidelines of the American Society of Echocardiography,12 before the procedure and at 1-month follow-up. All the examinations were stored in digital format and centrally analyzed by an independent experienced cardiologist unaware of clinical data using the Xcelera Cardiology Information System (Philips Medical Systems). The following measurements were obtained: aortic annulus diameter, left ventricular outflow tract diameter, left ventricular ejection fraction using the biplane Simpson method, the mean and peak transvalvular gradient estimated with the modified Bernoulli formula, valve effective orifice area calculated using the continuity equation, and the Doppler velocity index calculated as the left ventricular outflow tract velocity/transvalvular velocity ratio. The effective orifice area was indexed to the body surface area, and the presence of prosthesis-patient mismatch was defined as an indexed effective orifice area ≤ 0.85cm2/m2 (moderate between 0.65 to 0.85cm2/m2 and severe ≤ 0.65cm2/m2) if body mass index ≤ 30kg/m2 and indexed effective orifice area ≤ 0.70cm2/m2 (moderate between 0.60 to 0.70cm2/m2 and severe ≤ 0.60cm2/m2) if body mass index> 30kg/m2. The presence, degree, and type (transvalvular, paravalvular and total) of AoR were evaluated using a multiparametric approach and classified following the Valve Academic Research Consortium 2 recommendations13. In the presence of paravalvular AoR, the number of jets was also assessed. After hospital discharge, a follow-up visit was scheduled at 30 days and at 6 to 12 months and included an interview and physical examination by a cardiologist as well as transthoracic echocardiography. Procedural data, device success, and in-hospital events were prospectively recorded in a dedicated database and defined according to the Valve Academic Research Consortium 2 criteria. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee at both centers.
Statistical AnalysisCategorical variables are displayed as frequencies and comparisons between groups were performed using the chi-square or the Fisher exact test. Continuous variables are expressed as mean ± standard deviation or median [25th-75th interquartile range] and analyzed for normal distribution with the Shapiro-Wilk test. Comparisons were done using the Student t test or the Mann-Whitney U test depending on variable distribution. Differences were considered statistically significant when P<.05. All analyses were conducted using SPSS (SPSS Inc, Chicago, Illinos, United States) version 20.0 for windows statistical software.
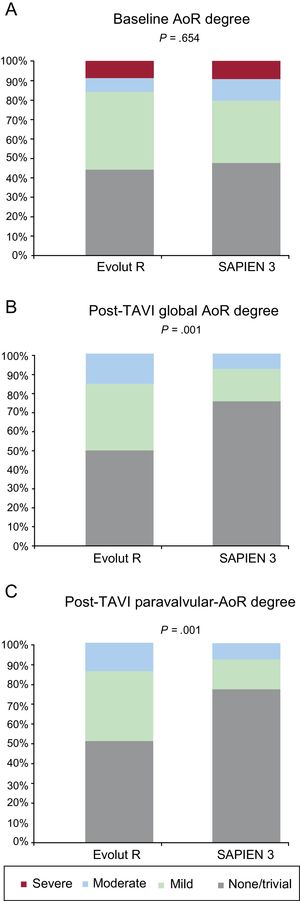
RESULTSThe mean age of population was 83±6-years and 52% were women. There were no differences in baseline clinical characteristics between the matched groups (Table 1). The main baseline echocardiography and MDCT characteristics according to valve type are depicted in Table 2. The matched variables as evaluated by MDCT were well balanced between groups (calcium score: EVR 2469±1764 vs S3 2504±2015; P=.91; aortic annulus diameter: EVR 24.6±2.5 vs S3 24.4±2.4mm; P=.68). There were also no differences regarding left ventricle dysfunction, stroke volume, severity of aortic stenosis, or rate and severity of AoR.
Baseline Characteristics, Overall and According to Transcatheter Valve Type
| All (N=144) | EVR (n=64) | S3 (n=80) | P | |
|---|---|---|---|---|
| Age, y | 83±6 | 84±5 | 82±6 | .11 |
| Female sex | 75 (52) | 37 (58) | 38 (48) | .30 |
| BSA, m2 | 1.70±0.2 | 1.68±0.2 | 1.72±0.2 | .14 |
| BMI, kg/m2 | 26.9±3.9 | 26.8±4.1 | 27.0±3.8 | .72 |
| Risk factors | ||||
| Diabetes mellitus | 53 (37) | 22 (34) | 31 (39) | .59 |
| Hyperlipidemia | 66 (46) | 30 (47) | 36 (45) | .82 |
| Hypertension | 115 (80) | 52 (81) | 63 (79) | .71 |
| Clinical features | ||||
| Prior MI | 31 (22) | 14 (22) | 17 (21) | .93 |
| Prior cardiac surgery | 14 (10) | 4 (6) | 10 (13) | .21 |
| COPD | 21 (15) | 10 (16) | 11 (14) | .75 |
| PVD | 10 (7) | 3 (5) | 7 (9) | .35 |
| CKD | 27 (19) | 15 (23) | 12 (15) | .20 |
| AF | 49 (34) | 19 (30) | 30 (38) | .32 |
| NYHA | .59 | |||
| I | 6 (4) | 2 (3) | 4 (5) | |
| II | 54 (38) | 21 (33) | 33 (42) | |
| III | 76 (53) | 38 (59) | 38 (48) | |
| IV | 7 (5) | 3 (5) | 4 (5) | |
| STS score, % | 6.0±5 | 5.8±5 | 6.2±5 | .62 |
AF, atrial fibrillation; BMI, body mass index; BSA, body surface area; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EVR, Evolut R; MI, myocardial infarction, PVD, peripheral vascular disease; NYHA, New York Heart Association; S3, SAPIEN 3; STS, Society of Thoracic Surgeons score.
Values are expressed as mean±standard deviation or No. (%).
Baseline Echocardiography and Computed Tomography Data Overall and According to Valve Type
| All (N=144) | EVR (n=64) | S3 (n=80) | P | |
|---|---|---|---|---|
| Computed tomography data | ||||
| Maximal aortic annulus diameter, mm | 26.2±3.2 | 26.3±2.9 | 26.2±3.5 | .90 |
| Minimal aortic annulus diameter, mm | 22.1±3.0 | 22.0±2.7 | 22.2±3.2 | .81 |
| Mean aortic annulus diameter, mm | 24.2±2.8 | 24.3±2.4 | 24.2±3.1 | .95 |
| Perimeter-derived diameter, mm | 24.5±2.4 | 24.6±2.5 | 24.4±2.4 | .68 |
| Eccentricity index | 15.3±9.2 | 15.6±10.6 | 15.1±8.0 | .074 |
| Aortic annulus area, mm2 | 455±96 | 459±93 | 451±98 | .61 |
| Aortic annulus perimeter, mm | 77±8 | 77±8 | 77±8 | .68 |
| Calcium score | 2488±1901 | 2469±1764 | 2504±2015 | .91 |
| Echocardiography data | ||||
| Heart rate, bpm | 72±11 | 71±10 | 72±11 | .47 |
| Ejection fraction <40% | 18 (13) | 5 (8) | 13 (17) | .12 |
| LVEDD, mm | 47±7 | 47±7 | 48±8 | .44 |
| LVESD, mm | 32±8 | 31±8 | 33±9 | .13 |
| Annulus diameter, mm | 22.7±2.5 | 22.7±2.6 | 22.7±2.5 | .87 |
| Stroke volume, mL | 48±14 | 48±10 | 48±15 | .96 |
| Peak aortic valve gradient, mmHg | 76±23 | 78±24 | 74±23 | .41 |
| Maximum velocity, m/s | 4.31±0.7 | 4.35±0.7 | 4.27±0.6 | .43 |
| Mean aortic valve gradient, mmHg | 46±14 | 47±14 | 45±15 | .54 |
| Effective orifice area, cm2 | 0.69±0.2 | 0.68±0.2 | 0.69±0.2 | .72 |
| Doppler velocity index | 0.21±0.1 | 0.21±0.1 | 0.21±0.1 | .89 |
| SPAP, mmHg | 43±15 | 43±14 | 42±15 | .80 |
| Aortic regurgitation | .65 | |||
| None/trace | 66 (46) | 28 (44) | 38 (48) | |
| Mild | 58 (40) | 29 (45) | 29 (36) | |
| Moderate | 15 (10) | 5 (8) | 10 (12) | |
| Severe | 5 (3.5) | 2 (3.1) | 3 (3.8) | |
| Moderate-severe aortic regurgitation | 20 (14) | 7 (11) | 13 (16) | .36 |
| Mitral regurgitation | .59 | |||
| None/trace | 61 (42) | 29 (45) | 32 (40) | |
| Mild | 57 (40) | 25 (39) | 32 (40) | |
| Moderate | 24 (17) | 10 (16) | 14 (18) | |
| Severe | 2 (1.4) | 0 (0.0) | 2 (2.5) | |
| Moderate-severe mitral regurgitation | 26 (18) | 10 (16) | 16 (20) | .50 |
bpm, beats per minute; EVR, Evolut R; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end systolic diameter; S3, SAPIEN 3; SPAP, systolic pulmonary artery pressure.
Values are expressed as mean±standard deviation or No. (%).
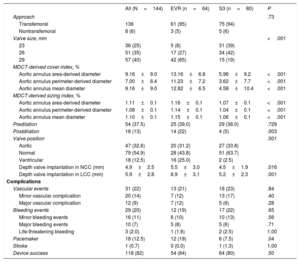
The main procedural and in-hospital events after TAVI are listed in Table 3. For a similar annulus dimension, the size of the transcatheter valve was greater in the EVR and consequently the degree of oversizing as assessed by the Cover and Sizing index was also greater in the EVR group (P=.001). Repositioning by resheathing the prosthesis was successfully attempted in 13 (20.3%) cases in the EVR group. The final implantation depth in noncoronary cusp was deeper in the EVR group than in the S3 group (5.5±3.0 vs 4.5±1.9; P=.016) (Table 3). The rate of balloon postdilation after valve implantation was higher in the EVR group (23%) than in the S3 group (5%), P=.002. There were no other differences between groups regarding periprocedural and in-hospital events, except for a higher incidence of new pacemaker implantation in the EVR group (18.8% vs 7.5%; P=.04).
Main Procedural Characteristics and 30-day Outcomes, Overall and According to Transcatheter Valve Type
| All (N=144) | EVR (n=64) | S3 (n=80) | P | |
|---|---|---|---|---|
| Approach | .73 | |||
| Transfemoral | 136 | 61 (95) | 75 (94) | |
| Nontransfemoral | 8 (6) | 3 (5) | 5 (6) | |
| Valve size, mm | <.001 | |||
| 23 | 36 (25) | 5 (8) | 31 (39) | |
| 26 | 51 (35) | 17 (27) | 34 (42) | |
| 29 | 57 (40) | 42 (65) | 15 (19) | |
| MDCT-derived cover index, % | ||||
| Aortic annulus area-derived diameter | 9.16±9.0 | 13.16±6.8 | 5.96±9.2 | <.001 |
| Aortic annulus perimeter-derived diameter | 7.00±8.4 | 11.23±7.2 | 3.62±7.7 | <.001 |
| Aortic annulus mean diameter | 9.16±9.0 | 12.82±6.5 | 4.58±10.4 | <.001 |
| MDCT-derived sizing index, % | ||||
| Aortic annulus area-derived diameter | 1.11±0.1 | 1.16±0.1 | 1.07±0.1 | <.001 |
| Aortic annulus perimeter-derived diameter | 1.08±0.1 | 1.14±0.1 | 1.04±0.1 | <.001 |
| Aortic annulus mean diameter | 1.10±0.1 | 1.15±0.1 | 1.06±0.1 | <.001 |
| Predilation | 54 (37.5) | 25 (39.0) | 29 (36.0) | .729 |
| Postdilation | 18 (13) | 14 (22) | 4 (5) | .003 |
| Valve position | .001 | |||
| Aortic | 47 (32.6) | 20 (31.2) | 27 (33.8) | |
| Normal | 79 (54.9) | 28 (43.8) | 51 (63.7) | |
| Ventricular | 18 (12.5) | 16 (25.0) | 2 (2.5) | |
| Depth valve implantation in NCC (mm) | 4.9±2.5 | 5.5±3.0 | 4.5±1.9 | .016 |
| Depth valve implantation in LCC (mm) | 5.9±2.8 | 6.9±3.1 | 5.2±2.3 | .001 |
| Complications | ||||
| Vascular events | 31 (22) | 13 (21) | 18 (23) | .84 |
| Minor vascular complication | 20 (14) | 7 (12) | 13 (17) | .40 |
| Major vascular complication | 12 (9) | 7 (12) | 5 (6) | .28 |
| Bleeding events | 29 (20) | 12 (19) | 17 (22) | .65 |
| Minor bleeding events | 16 (11) | 6 (10) | 10 (13) | .56 |
| Major bleeding events | 10 (7) | 5 (8) | 5 (6) | .71 |
| Life-threatening bleeding | 3 (2.0) | 1 (1.6) | 2 (2.5) | 1.00 |
| Pacemaker | 18 (12.5) | 12 (19) | 6 (7.5) | .04 |
| Stroke | 1 (0.7) | 0 (0.0) | 1 (1.3) | 1.00 |
| Device success | 118 (82) | 54 (84) | 64 (80) | .50 |
EVR, Evolut R; LCC, left coronary cusp; MDCT, multidetector computed tomography; NCC, noncoronary cusp; S3, SAPIEN 3.
Values are expressed as mean±standard deviation or No. (%).
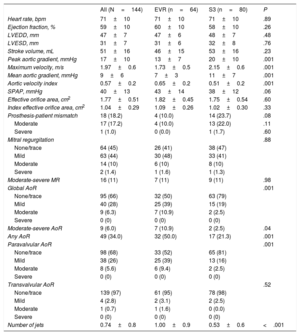
The echocardiographic data at 30-days after TAVI, overall and according to valve type, are listed in Table 4 and shown in Figure 2. The overall mean transprothesic gradient decreased from 46±14mmHg to 9±6mmHg; P<.001, and the mean effective orifice area increased from 0.69±0.2 to 1.77±0.51cm2 (P <.001) after TAVI. The EVR group showed a lower peak aortic gradient (EVR 13±7 vs S3 20±10mmHg; P <.001), lower mean aortic gradient (EVR 7±3 vs S3 11±7mmHg; P <.001), and a higher Doppler velocity index (EVR 0.65±0.15 vs S3 0.51±0.16; P <.001) compared with the S3 group. The incidence of prosthesis-patient mismatch tended to be higher in the S3 group (23.7% vs 10.0%; P=.082), with only one case of severe prosthesis-patient mismatch in the S3 group. The rate of moderate-severe AoR was higher in the EVR group (11%) than in the S3 group (2.5%) P=.04 (Figure 2). In addition, the rate of any paravalvular AoR (≥ mild) was higher in the EVR group (50%) than in the S3 group (21%) (P <.001), with a larger number of paravalvular jets (EVR 1.0±0.9 vs S3 0.5±0.6; P <.001). The final device success rate according to Valve Academic Research Consortium 2 criteria was similar in both groups (EVR 84% vs S3 80%; P=.50) (Table 3 and ). These hemodynamic results at 1 month remained similar in the imaging studies performed at 6-months’ follow-up (). The 12-month readmission rate due to heart failure (EVR 3.1% vs S3 5.0%; P=.576) and New York Heart Classification class was similar in the 2 groups, with most of the patients in New York Heart Classification class I to II (EVR 96.8% vs S3 96.1%; P=.374).
Doppler Echocardiographic Data at 1-month Follow-up After Transcatheter Aortic Valve Implantation According to Valve Type
| All (N=144) | EVR (n=64) | S3 (n=80) | P | |
|---|---|---|---|---|
| Heart rate, bpm | 71±10 | 71±10 | 71±10 | .89 |
| Ejection fraction, % | 59±10 | 60±10 | 58±10 | .26 |
| LVEDD, mm | 47±7 | 47±6 | 48±7 | .48 |
| LVESD, mm | 31±7 | 31±6 | 32±8 | .76 |
| Stroke volume, mL | 51±16 | 46±15 | 53±16 | .23 |
| Peak aortic gradient, mmHg | 17±10 | 13±7 | 20±10 | .001 |
| Maximum velocity, m/s | 1.97±0.6 | 1.73±0.5 | 2.15±0.6 | .001 |
| Mean aortic gradient, mmHg | 9±6 | 7±3 | 11±7 | .001 |
| Aortic velocity index | 0.57±0.2 | 0.65±0.2 | 0.51±0.2 | .001 |
| SPAP, mmHg | 40±13 | 43±14 | 38±12 | .06 |
| Effective orifice area, cm2 | 1.77±0.51 | 1.82±0.45 | 1.75±0.54 | .60 |
| Index effective orifice area, cm2 | 1.04±0.29 | 1.09±0.26 | 1.02±0.30 | .33 |
| Prosthesis-patient mismatch | 18 (18.2) | 4 (10.0) | 14 (23.7) | .08 |
| Moderate | 17 (17.2) | 4 (10.0) | 13 (22.0) | .11 |
| Severe | 1 (1.0) | 0 (0.0) | 1 (1.7) | .60 |
| Mitral regurgitation | .88 | |||
| None/trace | 64 (45) | 26 (41) | 38 (47) | |
| Mild | 63 (44) | 30 (48) | 33 (41) | |
| Moderate | 14 (10) | 6 (10) | 8 (10) | |
| Severe | 2 (1.4) | 1 (1.6) | 1 (1.3) | |
| Moderate-severe MR | 16 (11) | 7 (11) | 9 (11) | .98 |
| Global AoR | .001 | |||
| None/trace | 95 (66) | 32 (50) | 63 (79) | |
| Mild | 40 (28) | 25 (39) | 15 (19) | |
| Moderate | 9 (6.3) | 7 (10.9) | 2 (2.5) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| Moderate-severe AoR | 9 (6.0) | 7 (10.9) | 2 (2.5) | .04 |
| Any AoR | 49 (34.0) | 32 (50.0) | 17 (21.3) | .001 |
| Paravalvular AoR | .001 | |||
| None/trace | 98 (68) | 33 (52) | 65 (81) | |
| Mild | 38 (26) | 25 (39) | 13 (16) | |
| Moderate | 8 (5.6) | 6 (9.4) | 2 (2.5) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| Transvalvular AoR | .52 | |||
| None/trace | 139 (97) | 61 (95) | 78 (98) | |
| Mild | 4 (2.8) | 2 (3.1) | 2 (2.5) | |
| Moderate | 1 (0.7) | 1 (1.6) | 0 (0.0) | |
| Severe | 0 (0) | 0 (0) | 0 (0) | |
| Number of jets | 0.74±0.8 | 1.00±0.9 | 0.53±0.6 | <.001 |
AoR, aortic regurgitation; bpm, beats per minute; EVR, Evolut R; LVEDD, left ventricle end-diastolic diameter; LVESD, left ventricle end systolic diameter; MR, mitral regurgitation; S3, SAPIEN 3; SPAP, systolic pulmonary artery pressure.
Values are expressed as mean±standard deviation or No. (%).
The present study aimed to evaluate the hemodynamic profile of 2 newer-generation valves, the Edwards S3 and the Medtronic EVR transcatheter heart valves in a case-matched population. The principal findings from the present analysis are as follows: a) the EVR valve showed a better hemodynamic profile as assessed by lower peak and mean transprothesic gradients than the S3 at 1 and 6-month follow-up; b) the rate of moderate AoR was <12% in both groups, with no cases of severe AoR; and c) the rate of balloon postdilation and final rate of any and ≥ moderate paravalvular leakage were higher in the EVR group than in the S3.
Regarding the hemodynamic profile of the 2 valves, we found lower peak and mean gradients, and higher Doppler velocity index in the EVR valve than in the S3. This finding is in accordance with previous comparisons, in which the former self-expandable CoreValve also showed lower gradients than the SAPIEN XT valve.14,15 This was probably related to the supra-annular position of the leaflets in the self-expanding device, allowing lower resistance to the left ventricle outflow and gradients. The fact that the size of the valve was larger in the EVR group, leading to a higher degree of oversizing may be another reason for this difference in gradients. However, the native annulus size measured by MDCT was similar in both groups and the degree of oversizing was in accordance with current recommendations. Thus, in real life, the size of balloon-expandable valves is usually smaller than self-expandable valves for the same annulus dimension, with a lower degree of oversizing. In fact, a greater annulus dimension with balloon-expandable valves could translate into serious aortic complications. The long-term clinical benefit of this absolute difference of 4mmHg in mean gradient and the tendency of a higher rate of prosthesis-patient mismatch with S3, should be explored in long-term follow-up. Despite this difference in gradients, the global hemodynamic performance of the 2 valves was excellent and was similar to previous studies with these newer-generation valves.7–9,16,17 Because of the low profile of the stent frame, transcatheter valves are an interesting option for patients with a small annulus, reducing the risk of prosthesis-patient mismatch compared with surgical valves.18
Reducing paravalvular leakage is one of the main challenges with TAVI due to its association with worse outcomes, higher incidence compared with surgery, and the trend to use these devices in lower-risk populations.2–6 Initially, a trend toward a higher incidence of significant leakage with the first-generation self-expandable compared with the first-generation balloon-expandable valve was observed in some TAVI National Registries19,20 and multicenter studies.14 In a meta-analysis of observational studies, the rate of ≥ moderate AoR with first-generation transcatheter valves ranged from 0% to 47%, with a higher rate with self-expandable valve (16% vs 9.1%; P=.005) than with the balloon-expandable valve.2 Later, in the randomized CHOICE trial,15 a head-to-head comparison of the previous-generation CoreValve and SAPIEN XT showed a higher rate of device success in the SAPIEN XT group, driven mainly by a lower rate of moderate-severe AoR assessed by angiography (4.1% vs 18.3%; P <.001) that persisted in the 1-year follow-up.21 With second-generation valves, the reported rate of ≥ moderate AoR improved compared with previous generations,22,23 with an incidence <5%, but still greater than with surgery.5,6 In our case-matched study with independent echocardiography analysis, a 2.5% rate of moderate-severe AoR was found with the S3, similar to the ∼3.5% rate reported in previous registries across Europe and north America.5,16,24,25 We assumed this low rate of AoR is likely to result from a better annulus sizing, the presence of an external sealing cuff reducing the rate of paravalvular leakage, and the addition of a stable delivery platform optimizing valve positioning. With the EVR valve, information on the rate of AoR is limited to smaller cohorts. In the pivotal study of the EVR in 60 patients deemed to be at high to extreme risk for surgery,8 a rate of 3.4% of moderate-severe AoR was reported; the UK-Ireland EVR (n=264) registry, EVR US study (n=241), and the all-comers study by Perrin et al. (n=83) showed a 7.7%, 5.3% and 1.6% rate of moderate-severe AoR, respectively.9,17,26 In our real-life study, a slightly higher rate of moderate AoR (∼10%) was shown, but this rate was still lower than with previous-generation CoreValve.27 The newer design of the valve, in addition to the repositionable-retrievable nature of the system, may contribute to a proper and improved final positioning of the valve.
In the only study published to date directly comparing the S3 and the EVR valve, no differences were shown regarding the rate of moderate-severe AoR (2.5 vs 0%; P=.251, respectively) with very low rates of AoR in both groups.28 This was a single-center study with misbalanced groups, larger annulus diameter in the S3 group (with a preferable used of the S3 in patients with annulus size> 26mm), and without calcium adjustment, which is a well-known predictor of paravalvular AoR and need for balloon postdilation.29-31 In our matched cohort study, with valve sizing based on MDCT analysis and a similar degree of valve calcification in the 2 groups, EVR was associated with a higher degree of any and ≥ moderate paravalvular AoR. The greater radial force and the adaptability of S3 to the aortic annulus may be related to this lower rate of paravalvular AoR. Whether or not mild AoR increases long-term mortality continues under debate, ≥ moderate AoR is a well-accepted factor associated with poorer outcomes,3,4 and this absolute 8% difference in moderate AoR could have an impact on long-term clinical outcomes, especially in younger and less sick patients. In our analysis, these differences in the rate of AoR at 6 to 12 months’ follow-up remained. Thus, whether a further reduction in the paravalvular leakage severity over time with self-expandable systems, should be corroborated in future studies with longer follow-up. Importantly, the rate of significant paravalvular AoR was low in both groups with no cases of severe AoR, suggesting that recent changes in technology and valve sizing will further reduce the rate of paravalvular AoR in order to achieve good surgical results.
Although this study was not powered to detect differences in clinical events, no significant differences were found for in-hospital events between groups, except for the pacemaker implantation rate. In addition, the low rate of vascular complications and major bleeding reflected the benefit of the significant reduction in the sheath diameter with these 2 newer systems.
LimitationsThe main limitations of the present study are its limited sample size and observational nature. Patients were not randomized, but this was compensated by a very careful matching process between groups taking into account annulus size, and calcification of the aortic valve, which have been proven to correlate well with valve hemodynamics and the presence of paravalvular AoR. Assessment of AoR severity may be challenging, especially in the EVR group with a larger number of paravalvular jets and larger protrusion of the stent frame in the left ventricle outflow tract; nevertheless, a multiparametric echocardiographic approach ensures the accuracy of results with both devices. In addition, all the echocardiographic studies were evaluated by a single experienced echocardiographer unaware of clinical data, ensuring a uniform analysis. However, the presence of other potential confounding factors could not be excluded; therefore, these results need to be confirmed by future randomized clinical trials, with direct comparison between both transcatheter valves.
CONCLUSIONSIn this case-matched study, patients who underwent TAVI with the S3 valve had a lower rate of any and moderate paravalvular AoR, but higher residual gradients compared with patients with the EVR system at the 1- and 6-month follow-up. Long-term follow-up and larger scale multicenter experience will need to assess the possible effects of these observations on long-term clinical outcomes. In the meantime, these data suggest that in centers where both valves are available, S3 may be the preferred valve for patients with a higher risk of paravalvular leak, while EVR may be a better option for patients in whom hemodynamic could be jeopardized.
CONFLICTS OF INTERESTI.J. Amat-Santos has served as a proctor for Boston Scientific. L. Nombela-Franco has served as a proctor for Abbott and has received speaker honoraria from Edwards Lifesciences Inc.
- –
Transcatheter aortic valve implantation is recommended in patients with severe symptomatic aortic stenosis considered inoperable or at intermediate or high surgical risk. Paravalvular leak resulting in moderate-severe AoR is one the major concerns in early-generation transcatheter valves due to its association with increased long-term mortality. New-generation balloon-expandable S3 and self-expandable EVR were modified from their predecessors to further reduce the rate and degree of paravalvular leakage, increase device success, and reduce the rate of procedure-related complications.
- –
This case-matched study compares the hemodynamic performance of the new-generation balloon-expandable S3 vs self-expandable EVR in 2 centers with independent echocardiographic analysis. Transcatheter aortic valve implantation with the S3 valve showed a lower rate of any and moderate paravalvular AoR, but higher residual gradients when compared with patients with the EVR system. In-hospital and 30-day outcomes were similar between groups, except for a higher incidence of new pacemaker implantation in the EVR group.