There is a paucity of data comparing the left radial approach (LRA) and right radial approach (RRA) for percutaneous coronary intervention (PCI) in all-comers populations and performed by operators with different experience levels. Thus, we sought to compare the safety and clinical outcomes of the RRA and LRA during PCI in “real-world” patients with either stable angina or acute coronary syndrome (ACS).
MethodsTo overcome the possible impact of the nonrandomized design, a propensity score was calculated to compare the 2 radial approaches. The study group comprised 18 716 matched pairs with stable angina and 46 241 with ACS treated with PCI and stent implantation between 2014 and 2017 in 151 tertiary invasive cardiology centers in Poland (the ORPKI Polish National Registry).
ResultsThe rates of death and periprocedural complications were similar for the RRA and LRA in stable angina patients. A higher radiation dose was observed with PCI via the LRA in both clinical presentations (stable angina: 1067.0±947.1 mGy vs 1007.4±983.5 mGy, P=.001; ACS: 1212.7±1005.5 mGy vs 1053.5±1029.7 mGy, P=.001). More contrast was used in LRA procedures but only in ACS patients (174.2±75.4mL vs 167.2±72.1mL, P=.001). Furthermore, periprocedural complications such as coronary artery dissection (0.16% vs 0.09%, P=.008), no-reflow phenomenon (0.65% vs 0.49%, P=.005), and puncture site bleeding (0.09% vs 0.05%, P=.04) were more frequently observed with the LRA in ACS patients. There was no difference in mortality between the 2 groups (P=.90).
ConclusionsOur finding of poorer outcomes with the LRA may be related to lower operator experience with this approach. While both the LRA and RRA are safe in the setting of stable angina, the LRA was associated with a higher rate of periprocedural complications during PCI in ACS patients.
Keywords
The use of the radial approach for percutaneous coronary intervention (PCI) has been linked to reduced mortality and bleeding complications compared with the femoral approach.1–6 The right radial approach (RRA) has been widely adopted as the approach of choice for percutaneous interventions, despite more marked anatomical variations and technical difficulties.7,8 Conversely, the left radial approach (LRA) can potentially improve catheter manipulation due to a more favorable anatomy and is considered similar to the femoral approach.8–10 Nonetheless, the LRA is less used by invasive cardiologists.8,9
There is a paucity of data comparing the clinical outcomes of left and right radial artery use for PCI in all-comers populations and performed by operators with different experience levels. It thus remains unclear which artery is a more favorable choice for radial access. However, a growing body of clinical evidence suggests the advantage of the LRA over the RRA in terms of the procedural time, radiation dose, and rate of cerebrovascular complications.8,11–14 Thus, we sought to compare the safety and clinical outcomes of the RRA and LRA during PCI in an unselected cohort of patients with either stable angina (SA) or acute coronary syndrome (ACS) based on data from the Polish National PCI Registry (ORPKI).
METHODSThe ORPKI is a national Polish registry that collects data on all interventional cardiology procedures performed in Poland.15–19 This registry is endorsed by the Polish Association of Cardiovascular Interventions of the Polish Cardiac Society and operated by Jagiellonian University Medical College in Krakow.20 No personal data are stored in the database. For this prospective, observational study, we evaluated data from 151 invasive cardiology centers in Poland from January 2014 to December 2017. The study included 330 450 consecutive patients undergoing PCI with stent implantation via the RRA or LRA.
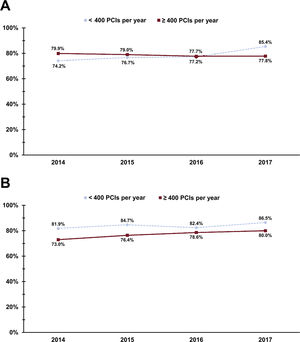
The procedures were conducted using the RRA and LRA in 255 866 (77.4%) and 74 584 (22.6%) patients, respectively. The frequency of RRA use between 2014 and 2017 in centers performing <400 and ≥ 400 PCIs per year for both SA and ACS is presented in figure 1. After propensity score matching, the analysis included 18 716 matched SA patients and 46 241 matched ACS patients treated with PCI via the RRA or LRA. The patient flow chart is presented in figure 2.
All PCIs were performed by operators with different experience and dexterity levels with the radial approach. The total radial experience was determined separately for each operator based on unique identification numbers in the registry. It was calculated as the total number of PCIs performed via the radial approach between 2014 and 2017. The access site for procedures as well as target lesion selection and treatment technique were at the operator's discretion. The access site for each procedure was determined as the site of successful vascular entry. There were 1529 procedures (0.46%) with an unidentified access site. Furthermore, the vascular access site was changed in 159 patients with SA (0.17%) and in 3619 patients with ACS (1.5%). All procedures performed with unidentified or switched access sites were excluded from the analysis. In addition, data on complexity and lesion type were not collected.
All procedures were performed in accordance with local PCI standards and European Society of Cardiology guidelines wherever applicable. All intraprocedural complications were prospectively recorded. Periprocedural mortality was defined as all-cause death during PCI up to transfer from the catheterization laboratory to either the cardiology department or intensive care unit. Bleeding complications were defined homogeneously in all centers as any overt, actionable sign of hemorrhage (eg, more bleeding than would be expected for the clinical circumstance, including bleeding found by imaging alone) that did not meet the criteria for type 3, 4, or 5 bleeding21 but did meet at least 1 of the following criteria: a) requiring nonsurgical, medical intervention by a health care professional, b) leading to hospitalization or increased level of care, or c) prompting evaluation. Stroke was diagnosed by local physicians. Specified data on the type of stroke and neurological outcomes were not provided. No-reflow was defined as failure to restore optimal myocardial perfusion through the coronary artery without angiographic evidence of mechanical vessel obstruction, dissection, spasm, or distal embolism (Thrombolysis in Myocardial Infarction grade ≤ 2 flow). Cardiac arrest was diagnosed in the absence of organized electrical activity in the myocardium with no consistent contraction of the ventricles, resulting in inability of the heart to generate adequate cardiac output. This definition included both resuscitated patients and those with sudden cardiac arrest resulting in death. Adverse events were identified at the operator's discretion in accordance with the definitions in current European Society of Cardiology guidelines.22 No evaluation of follow-up was performed after patients were discharged from hospital.
All patients provided signed informed consent for each procedure. The study complied with ethical principles for clinical research based on the Declaration of Helsinki with later amendments. There was no financial support for this registry.
Statistical analysisTo overcome a possible impact of the nonrandomized design, a propensity score was calculated using a multivariate logistic regression model with access site (right vs left radial) as the dependent variable. To reduce analytical biases, site volume ≥ 400 PCIs and all available baseline characteristics were set as covariates, including sex, age, weight, diabetes mellitus, previous stroke, previous myocardial infarction, previous PCI, previous coronary artery bypass grafting, smoking status, hypertension, chronic kidney disease, psoriasis, periprocedural treatment (acetylsalicylic acid, P2Y12 inhibitors, unfractionated heparin, low-molecular-weight heparin), baseline clinical data, indication for the ACS group (ST-segment elevation myocardial infarction, non-ST-segment elevation myocardial infarction, unstable angina), and baseline Thrombolysis in Myocardial Infarction flow. A caliper cutoff was used to obtain satisfactory balancing by ensuring that the standardized differences for all confounders were estimated to be less than 10%. Patients were matched in a 1:1 proportion and unpaired patients were not included in the matched-paired analysis.
The analysis was performed separately for both SA and ACS and included standard descriptive statistics. Quantitative variables are reported as mean±standard deviation. Categorical variables are described as number and percentage. To compare groups before matching, we used the Mann-Whitney U test (for nonnormally distributed data) or the t test (for normally distributed data) for continuous variables and the Fisher exact test or Pearson chi-square test for categorical (nominal and dichotomous) variables. The normality of the data was assessed with the Kolmogorov-Smirnov test with Lilliefors correction. Matched pairs were compared with the Wilcoxon signed-rank test (for differences in nonnormally distributed data) or the paired t test (for differences in normally distributed data) for continuous variables and the McNemar-Bowker test for categorical (nominal) variables.
The P values for the periprocedural results of percutaneous coronary interventions after propensity score matching were adjusted using the Benjamini, Hochberg, and Yekutieli FDR-controlling procedure. The analysis was performed in the “as-treated” manner. In addition, multivariate logistic regression analysis was performed to identify possible determinants and independent predictors of left or right artery use for PCI. Backward selection in logistic regression analysis with a probability value for covariates to enter the model was set at the .05 level. All available preprocedural (baseline) variables were included to build the final model. Due to the large number of cases, it was possible to completely control for confounding as much as possible and to simultaneously reduce the possibility of overfitting and numerically unstable estimates.
Results are presented as odds ratios (ORs) with 95% confidence intervals (95%CIs). Due to the large amount of data and relatively low rates of missing data, no data imputation methods were used. All statistical analyses were performed with JMP version 14.2.0 (SAS Institute Inc, United States).
RESULTSComplete baseline clinical and demographic data are presented in table 1. All presented data were calculated using matched pairs. Details of the angiographic indications for PCI and both antiplatelet and antithrombotic therapy during the procedure are presented for all included patients and separately for SA and ACS in table 2, table 3, and table 4, respectively.
Baseline characteristics before and after propensity score matching
| Variable | Left radial access (n=74 584) | Right radial access (n=255 866) | P |
|---|---|---|---|
| Male sex | 50 911 (68.4) | 177 159 (69.8) | .001 |
| Weight, kg | 81.3±16.0 | 81.3±17.6 | .03 |
| Age, y | 67.4±10.7 | 66.6±10.7 | .004 |
| Diabetes mellitus | 18 175 (24.4) | 60 671 (23.7) | .002 |
| Previous stroke | 2358 (3.2) | 7875 (3.1) | .20 |
| Previous MI | 24 606 (33.0) | 74 487 (29.1) | .001 |
| Previous CABG | 7704 (10.3) | 5606 (2.2) | .001 |
| Previous PCI | 28 808 (38.6) | 91 466 (35.8) | .001 |
| Smoking | 14 125 (19.0) | 53 667 (21.0) | .001 |
| Hypertension | 53 786 (72.1) | 181 389 (70.9) | .001 |
| Chronic kidney disease | 3823 (5.1) | 11 813 (4.6) | .001 |
| Chronic obstructive pulmonary disease | 1438 (2.5) | 5281 (2.6) | .50 |
| Psoriasis | 319 (0.4) | 1031 (0.4) | .30 |
| Cardiac arrest at baseline | 547 (1.0) | 1946 (0.9) | .40 |
| Data after propensity score matching in stable angina | Left radial access (n=18 716) | Right radial access (n=18 716) | P |
|---|---|---|---|
| Male sex | 13 016 (69.5) | 13 281 (71.0) | .10 |
| Weight, kg | 81.9±15.6 | 81.9±17.0 | .70 |
| Age, y | 67.3±9.7 | 67.0±9.6 | .10 |
| Diabetes mellitus | 4999 (26.7) | 4895 (26.2) | .20 |
| Previous stroke | 554 (3.0) | 559 (3.0) | .90 |
| Previous MI | 7955 (42.5) | 7996 (42.7) | .70 |
| Previous CABG | 2090 (11.2) | 1930 (10.3) | .10 |
| Previous PCI | 10 104 (54.0) | 10 334 (55.2) | .001 |
| Smoking | 2895 (15.5) | 2771 (14.8) | .10 |
| Hypertension | 14 233 (76.1) | 14 285 (76.3) | .50 |
| Chronic kidney disease | 1034 (5.5) | 985 (5.3) | .30 |
| Chronic obstructive pulmonary disease | 451 (2.8) | 394 (2.4) | .20 |
| Psoriasis | 67 (0.4) | 59 (0.3) | .50 |
| Cardiac arrest at baseline | - | - | - |
| Data after propensity score matching in acute coronary syndrome | Left radial access (n=46 241) | Right radial access (n=46 241) | P |
|---|---|---|---|
| Male sex | 68.2 (31 520) | 68.6 (31 723) | .10 |
| Weight, kg | 81.0±15.9 | 81.0±17.4 | .90 |
| Age, y | 67.2±11.1 | 67.0±11.0 | .10 |
| Diabetes mellitus | 10 791 (23.3) | 10 722 (23.2) | .60 |
| Previous stroke | 1474 (3.2) | 1459 (3.2) | .80 |
| Previous MI | 13 178 (28.5) | 12 724 (27.5) | .003 |
| Previous CABG | 3007 (6.5) | 2906 (6.3) | .10 |
| Previous PCI | 14 894 (32.2) | 14 425 (31.2) | .001 |
| Smoking | 9934 (21.5) | 10 388 (22.5) | .10 |
| Hypertension | 32 990 (71.3) | 32 975 (71.3) | .90 |
| Chronic kidney disease | 2221 (4.8) | 2340 (5.1) | .10 |
| Chronic obstructive pulmonary disease | 798 (2.4) | 943 (2.6) | .10 |
| Cardiac arrest at baseline | 494 (1.3) | 501 (1.3) | .50 |
CABG, coronary artery bypass grafting; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Data are expressed as No. (%) or mean±standard deviation.
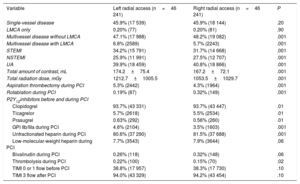
Percutaneous coronary intervention details after propensity score matching in stable angina
| Variable | (n=18 716) | Right radial access (n=18 716) | P |
|---|---|---|---|
| Single-vessel disease | 46.7% (5063) | 53.5% (6825) | .001 |
| LMCA only | 0.3% (30) | 0.3% (33) | .80 |
| Multivessel disease without LMCA | 46.2% (5008) | 41.5% (5292) | .001 |
| Multivessel disease with LMCA | 6.8% (739) | 4.6% (587) | .001 |
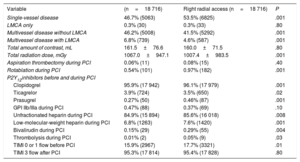
| Total amount of contrast, mL | 161.5±76.6 | 160.0±71.5 | .80 |
| Total radiation dose, mGy | 1067.0±947.1 | 1007.4±983.5 | .001 |
| Aspiration thrombectomy during PCI | 0.06% (11) | 0.08% (15) | .40 |
| Rotablation during PCI | 0.54% (101) | 0.97% (182) | .001 |
| P2Y12inhibitors before and during PCI | |||
| Clopidogrel | 95.9% (17 942) | 96.1% (17 979) | .001 |
| Ticagrelor | 3.9% (724) | 3.5% (650) | .02 |
| Prasugrel | 0.27% (50) | 0.46% (87) | .001 |
| GPI IIb/IIIa during PCI | 0.47% (88) | 0.37% (69) | .10 |
| Unfractionated heparin during PCI | 84.9% (15 894) | 85.6% (16 018) | .008 |
| Low-molecular-weight heparin during PCI | 6.8% (1263) | 7.6% (1420) | .001 |
| Bivalirudin during PCI | 0.15% (29) | 0.29% (55) | .004 |
| Thrombolysis during PCI | 0.01% (2) | 0.05% (9) | .03 |
| TIMI 0 or 1 flow before PCI | 15.9% (2967) | 17.7% (3321) | .01 |
| TIMI 3 flow after PCI | 95.3% (17 814) | 95.4% (17 828) | .80 |
GPI IIb/IIIa, glycoprotein IIb/IIIa inhibitors; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Data are expressed as No. (%) or mean±standard deviation.
Percutaneous coronary intervention details after propensity score matching in acute coronary syndrome
| Variable | Left radial access (n=46 241) | Right radial access (n=46 241) | P |
|---|---|---|---|
| Single-vessel disease | 45.9% (17 539) | 45.9% (18 144) | .20 |
| LMCA only | 0.20% (77) | 0.20% (81) | .90 |
| Multivessel disease without LMCA | 47.1% (17 988) | 48.2% (19 082) | .001 |
| Multivessel disease with LMCA | 6.8% (2589) | 5.7% (2243) | .001 |
| STEMI | 34.2% (15 791) | 31.7% (14 668) | .001 |
| NSTEMI | 25.9% (11 991) | 27.5% (12 707) | .001 |
| UA | 39.9% (18 459) | 40.8% (18 866) | .001 |
| Total amount of contrast, mL | 174.2±75.4 | 167.2±72.1 | .001 |
| Total radiation dose, mGy | 1212.7±1005.5 | 1053.5±1029.7 | .001 |
| Aspiration thrombectomy during PCI | 5.3% (2442) | 4.3% (1964) | .001 |
| Rotablation during PCI | 0.19% (87) | 0.32% (149) | .001 |
| P2Y12inhibitors before and during PCI | |||
| Clopidogrel | 93.7% (43 331) | 93.7% (43 447) | .01 |
| Ticagrelor | 5.7% (2618) | 5.5% (2534) | .01 |
| Prasugrel | 0.63% (292) | 0.56% (260) | .01 |
| GPI IIb/IIIa during PCI | 4.6% (2104) | 3.5% (1603) | .001 |
| Unfractionated heparin during PCI | 80.6% (37 290) | 81.5% (37 688) | .001 |
| Low-molecular-weight heparin during PCI | 7.7% (3543) | 7.9% (3644) | .06 |
| Bivalirudin during PCI | 0.26% (118) | 0.32% (148) | .06 |
| Thrombolysis during PCI | 0.22% (100) | 0.15% (70) | .02 |
| TIMI 0 or 1 flow before PCI | 38.8% (17 957) | 38.3% (17 730) | .10 |
| TIMI 3 flow after PCI | 94.0% (43 329) | 94.2% (43 454) | .10 |
GPI IIb/IIIa, glycoprotein IIb/IIIa inhibitors; LMCA, left main coronary artery; NSTEMI, non-ST-segment elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; UA, unstable angina.
Data are expressed as No. (%) or mean±standard deviation.
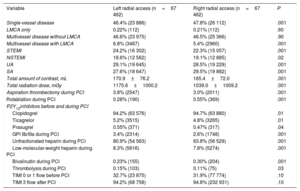
Percutaneous coronary intervention details after propensity score matching for all included patients
| Variable | Left radial access (n=67 462) | Right radial access (n=67 462) | P |
|---|---|---|---|
| Single-vessel disease | 46.4% (23 886) | 47.8% (26 112) | .001 |
| LMCA only | 0.22% (112) | 0.21% (112) | .60 |
| Multivessel disease without LMCA | 46.6% (23 975) | 46.5% (25 366) | .90 |
| Multivessel disease with LMCA | 6.8% (3487) | 5.4% (2960) | .001 |
| STEMI | 24.2% (16 302) | 22.3% (15 057) | .001 |
| NSTEMI | 18.6% (12 562) | 19.1% (12 885) | .02 |
| UA | 29.1% (19 645) | 28.5% (19 229) | .001 |
| SA | 27.6% (18 647) | 29.5% (19 882) | .001 |
| Total amount of contrast, mL | 170.9±76.2 | 165.4±72.0 | .001 |
| Total radiation dose, mGy | 1175.6±1000.2 | 1039.0±1009.2 | .001 |
| Aspiration thrombectomy during PCI | 3.8% (2547) | 3.0% (2011) | .001 |
| Rotablation during PCI | 0.28% (190) | 0.55% (369) | .001 |
| P2Y12inhibitors before and during PCI | |||
| Clopidogrel | 94.2% (63 576) | 94.7% (63 880) | .01 |
| Ticagrelor | 5.2% (3515) | 4.8% (3265) | .01 |
| Prasugrel | 0.55% (371) | 0.47% (317) | .04 |
| GPI IIb/IIIa during PCI | 3.4% (2314) | 2.6% (1748) | .001 |
| Unfractionated heparin during PCI | 80.9% (54 563) | 83.8% (56 529) | .001 |
| Low-molecular-weight heparin during PCI | 8.3% (5616) | 7.8% (5274) | .001 |
| Bivalirudin during PCI | 0.23% (155) | 0.30% (204) | .001 |
| Thrombolysis during PCI | 0.15% (103) | 0.11% (75) | .03 |
| TIMI 0 or 1 flow before PCI | 32.7% (23 875) | 31.9% (77 774) | .10 |
| TIMI 3 flow after PCI | 94.2% (68 758) | 94.8% (232 931) | .10 |
GPI IIb/IIIa, glycoprotein IIb/IIIa inhibitors; LMCA, left main coronary artery; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; SA, stable angina; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina; TIMI: Thrombolysis in Myocardial Infarction
Data are expressed as No. (%) or mean±standard deviation.
After propensity score matching, higher radiation doses were observed for PCI with left radial artery use in both SA and ACS settings (LRA vs RRA, respectively: 1067.0±947.1 mGy vs 1007.4±983.5 mGy for stable angina, P=.001; 1212.7±1005.5 mGy vs 1053.5±1029.7 mGy for ACS, P=.001) (table 2 and table 3). However, a higher total amount of contrast was observed for the LRA vs the RRA only in ACS procedures (174.2±75.4mL vs 167.2±72.1mL, P=.001). Furthermore, there were higher radiation doses and a total amount of contrast in PCI via the LRA in all included patients (radiation: 1175.6±1000.2 mGy vs 1039.0±1009.2 mGy, P=.001; contrast: 170.9±76.2mL vs 165.4±72.0mL, P=.001).
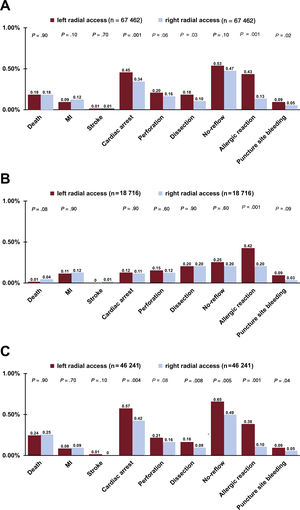
A similar prevalence of periprocedural complications was observed during PCI in SA patients for both the LRA and RRA. However, an allergic reaction was more frequent in procedures performed with the LRA than in those performed with the RRA. In addition, a trend toward increased risk of bleeding complications was observed in the LRA group. Conversely, a numerically but nonsignificantly higher rate of periprocedural mortality was associated with the RRA in SA procedures (0.01% vs 0.04%, P=.08) (figure 3). In contrast, the LRA was linked with more detrimental outcomes in ACS patients vs the RRA. Allergic reaction, cardiac arrest, puncture site bleeding, coronary artery dissection, and no-reflow phenomenon were more frequently reported in ACS patients during PCI conducted with the LRA. Furthermore, there was a nonsignificant trend toward a higher rate of coronary artery perforation in procedures performed in ACS patients via the LRA (P=.08) (figure 3). No differences in mortality were observed between the 2 groups of ACS patients (P=.90) (figure 3). Furthermore, allergic reaction, cardiac arrest, puncture site bleeding, and coronary artery dissection occurred more frequently in all included patients during PCI via the LRA. No statistically significant trend toward increased risk of coronary artery perforation was noted in PCI with the LRA (P=.06). There were no differences in mortality between the LRA and RRA in all included patients (P=.90). The periprocedural results of PCI are presented for all included patients and separately for SA and ACS in figure 3.
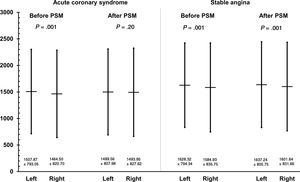
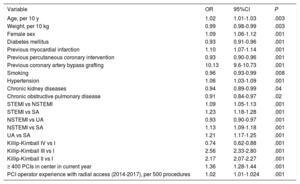
In multivariate analysis, previous coronary artery bypass grafting was the strongest independent predictor of the LRA being chosen for PCI. Furthermore, invasive cardiologists from large centers performing ≥ 400 PCIs per year were more likely to select the LRA for procedures (table 5). Invasive cardiologists with similar radial experience performed PCI via both the LRA and RRA in ACS patients. However, slightly more experienced radial operators performed PCI via the LRA in SA patients (figure 4).
Independent predictors of left radial artery use for percutaneous coronary intervention
| Variable | OR | 95%CI | P |
|---|---|---|---|
| Age, per 10 y | 1.02 | 1.01-1.03 | .003 |
| Weight, per 10 kg | 0.99 | 0.98-0.99 | .003 |
| Female sex | 1.09 | 1.06-1.12 | .001 |
| Diabetes mellitus | 0.93 | 0.91-0.96 | .001 |
| Previous myocardial infarction | 1.10 | 1.07-1.14 | .001 |
| Previous percutaneous coronary intervention | 0.93 | 0.90-0.96 | .001 |
| Previous coronary artery bypass grafting | 10.13 | 9.6-10.73 | .001 |
| Smoking | 0.96 | 0.93-0.99 | .008 |
| Hypertension | 1.06 | 1.03-1.09 | .001 |
| Chronic kidney diseases | 0.94 | 0.89-0.99 | .04 |
| Chronic obstructive pulmonary disease | 0.91 | 0.84-0.97 | .02 |
| STEMI vs NSTEMI | 1.09 | 1.05-1.13 | .001 |
| STEMI vs SA | 1.23 | 1.18-1.28 | .001 |
| NSTEMI vs UA | 0.93 | 0.90-0.97 | .001 |
| NSTEMI vs SA | 1.13 | 1.09-1.18 | .001 |
| UA vs SA | 1.21 | 1.17-1.25 | .001 |
| Killip-Kimball IV vs I | 0.74 | 0.62-0.88 | .001 |
| Killip-Kimball III vs I | 2.56 | 2.33-2.80 | .001 |
| Killip-Kimball II vs I | 2.17 | 2.07-2.27 | .001 |
| ≥ 400 PCIs in center in current year | 1.36 | 1.28-1.44 | .001 |
| PCI operator experience with radial access (2014-2017), per 500 procedures | 1.02 | 1.01-1.024 | .001 |
95%CI, 95% confidence interval; NSTEMI, non-ST-segment elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; SA, stable angina; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina.
OR> 1 favors left radial artery use over right radial use for PCI.
Experience of invasive cardiologists expressed as the total number of percutaneous coronary interventions via the radial approach between 2014 and 2017. Data are presented before and after propensity score matching for stable angina and acute coronary syndrome. PSM, propensity score matching.
The results of this study demonstrate that the LRA and RRA are equally safe and effective in the setting of SA. However, a higher radiation dose was observed in PCI via the LRA, regardless of the clinical presentation. Likewise, more contrast was used in all LRA procedures and in the setting of ACS. Compared with the RRA, LRA use was associated with more detrimental periprocedural outcomes in all included patients and in the ACS subgroup. The present results might be explained by the generally lower experience with the LRA in emergency scenarios. To the best of our knowledge, the current study is the largest multicenter registry to provide insight into the left and right radial approaches in both SA and ACS in contemporary clinical cardiology.
Previous studies comparing the LRA and RRA have yielded conflicting results. Several reports suggested superiority of the left over right radial artery in terms of the procedural time, radiation dose, and rate of cerebrovascular complications.11–14 However, the TALENT study23 found similar procedural and fluoroscopy times for the 2 approaches in coronary diagnostic procedures performed by well-trained operators. Slight reductions were observed in fluoroscopy times with the LRA in older patients and for operators in training. However, another randomized study did not confirm this finding.24 Furthermore, a previous randomized trial comparing the 2 radial approaches for coronary angiography determined similar safety profiles and no differences in the amount of contrast or radiation dose.25 These findings were consistent with the analysis in the STEMI subpopulation. No differences were reported between the 2 radial approaches in contrast volume, fluoroscopy time, periprocedural vascular complications, and stroke/transient ischemic attack (TIA) or death.26
Conversely, data from another STEMI cohort outlined a lower radiation dose with the LRA with no difference in contrast volume between the 2 approaches.27 Furthermore, a recent analysis suggested that the LRA was associated with shorter fluoroscopy and procedural times compared with right radial artery use during PCI in ACS patients. However, no difference was found in the amount of contrast used.28 Another study29 based on propensity score matching in 1100 patients suggested an association of the RRA with a larger contrast volume and longer fluoroscopy time. However, in-hospital and 12-month clinical outcomes were similar in the 2 groups. Finally, a recent meta-analysis involving 3210 patients failed to detect a difference in clinical outcomes between the 2 radial access sites.30 In contrast, further accumulation of data updated the outcome and demonstrated an advantage of the LRA in terms of fluoroscopy time and contrast use vs the right radial group for both diagnostic and interventional coronary procedures. Remarkably, body mass index was proposed to be a potential source of heterogeneity in outcomes between trials.31 The latest pooled meta-analysis of 6450 patients showed a longer fluoroscopy time and higher contrast use with the RRA. Similar rates of access site complications and stroke were observed with the 2 radial approaches.9 However, all of these studies had a relatively small sample size that was insufficient to detect differences in clinical outcomes. In addition, the abovementioned meta-analyses included studies without unified end point definitions. Moreover, the data were from different health care systems and countries and were insufficiently homogeneous.
Our results contradict those of most contemporary studies. The higher radiation dose, contrast volume, and rate of periprocedural complications might be linked to lower dexterity in LRA use. In our database, invasive cardiologists chose the RRA more than 3 times more frequently than the LRA. Furthermore, more complex procedures were probably undertaken with the left radial artery approach because the independent predictors of the selection of this access site were previous coronary artery bypass grafting and worse clinical settings (higher rate of multivessel disease with left main coronary artery disease and more patients with STEMI), as well as PCI in high-volume centers (table 3, table 4, and table 5).
Furthermore, total operator experience and radial dexterity level were not considered in most previous studies. In our study, PCIs in the setting of ACS were performed by equally experienced invasive cardiologists in both the LRA and RRA groups. However, slightly more experienced radial operators performed PCI with the LRA in SA patients (figure 4). Furthermore, there was a slightly higher rate of Thrombolysis in Myocardial Infarction (TIMI) 0 or 1 flow before PCI in the RRA group of SA patients. These factors might be related to a trend toward increased mortality in SA during procedures performed with the RRA. Importantly, a recent study demonstrated a higher rate of stroke during PCIs performed by operators with lower experience with the radial access.15 In that multivariate logistic regression analysis, the percent of PCIs performed with radial access per operator (OR, 0.981 per 1% increase; 95%CI, 0.967-0.997; P=.02) was considered an independent predictor of periprocedural stroke.15 In contrast, recent data from the ACCOAST trial demonstrated no impact of radial access on the risk of stroke.32 In our analysis, a low rate of periprocedural stroke was observed, with no differences between groups. However, several factors might have an impact on favorable outcomes in the LRA. Various analyses showed a higher rate of tortuosity in the right than left subclavian artery.7,9,13 Thus, left radial artery use may facilitate direct access to the ascending aorta or left internal mammary artery and allow quicker device delivery.7,13 In addition, catheter maneuvers with the LRA are considered very similar to those with the femoral approach.7,13 These differences might partially explain some reports of a higher radiation dose and contrast volume used for procedures performed via the RRA. However, while most of the studies recorded fluoroscopy time, this measurement has limited ability to assess real radiation exposure7,9,13 and is considered merely a general indicator of the X-ray radiation produced. Accordingly, there is no direct association between operator exposure and absorption in particular parts of the body, as well as above and below the surface of the apron.9,13 Dose-area product or air kerma are much more clinically relevant measures of radiation exposure.12,13,30 Notably, patient weight is also a crucial variable related to operator exposure. Unfortunately, most of these parameters were not included in the abovementioned studies. In our study, no difference in body mass was observed between the LRA and RRA groups for both SA and ACS (table 1). However, a higher radiation dose was observed in PCI via the LRA, regardless of clinical presentation.
In everyday clinical practice, the RRA is traditionally favored by most invasive cardiologists, primarily because the right side of the patient provides more suitable conditions for operators and because radial compression devices are mainly designed for the right wrist.7,9,13,30 The most important impediment to the widespread adoption of the LRA is related to the uncomfortable position required to reach the left forearm. Shorter operators will find it especially cumbersome to lean over patients, particularly obese patients. Furthermore, invasive cardiologists performing PCI via the LRA are exposed to higher radiation doses related to this position. This factor might partially explain a higher radiation dose in PCIs performed via the LRA. Both radial approaches are clinically relevant but the radial artery puncture side might have major implications in routine clinical practice. The anatomical advantage of the LRA might be an attractive alternative for invasive cardiologists in training. However, this approach might be associated with an increased risk of complications. Further studies with long-term observation should be performed to help to identify the optimal radial access approach.
LimitationsThe results of this study should be interpreted in light of several limitations. The most important limitation is the nonrandomized design, with all of its intrinsic biases. The possibility of unmeasured confounders influencing the outcome cannot be ruled out. However, a propensity score matching procedure was used to surmount this limitation. Detailed distributions and the experience levels of both radial approaches per center and each invasive cardiologist were not analyzed. Thus, some center- or operator-related factors might have influenced outcomes. However, we presented data for the total radial PCI experience and for high-volume centers performing ≥ 400 PCIs per year. Notably, the decision regarding the access site for intervention and treatment was at the operator's discretion. Furthermore, some clinical data were lacking. We did not record data on the prevalence of subclavian tortuosity or anatomical variations on the right or left side. Also not recorded were the size of the vascular sheaths used during the PCI and the use of closure devices, as well as the duration of the procedure and data after hospital discharge. Long-term end points might be crucial for a comprehensive evaluation of the superiority of left or right radial artery use. Because there was a deficiency in some clinical data, propensity score matching might not be sufficient to exclude the impact of hypothetical unmeasured factors. Thus, the results should be considered exploratory and hypothesis-generating. Despite all of these limitations, the present data reflect the experience of a study with an all-comers design. Thus, the findings could be extrapolated to the general population.
CONCLUSIONSBoth radial approaches appear to be equally safe and effective in the setting of SA. Patients treated via the LRA show higher radiation doses and total contrast amount, regardless of clinical presentation. An increased incidence of periprocedural complications during PCI with the LRA might be explained by the generally lower experience with this approach.
CONFLICTS OF INTERESTAll authors declare no conflicts of interest.
- -
Although the use of the radial approach for PCI has been linked with lower mortality and fewer bleeding complications vs the femoral approach, it remains unclear which artery is a more favorable choice for radial access.
- -
There is a paucity of data comparing clinical outcomes between the LRA and RRA for PCI in all-comers populations and performed by operators with different experience levels, and previous studies comparing the LRA and RRA have yielded conflicting results.
- -
However, a growing body of clinical evidence suggests the advantage of the LRA over the RRA in reducing the procedural time, radiation dose, and rate of cerebrovascular complications.
- -
The current study is the largest multicenter registry to compare the LRA and RRA in SA and ACS patients in contemporary clinical cardiology.
- -
The LRA and RRA are equally safe and effective in the setting of SA but, in contrast to most recent data, a higher radiation dose and increased incidence of periprocedural complications were observed in PCI via the LRA, regardless of clinical presentation.
- -
Moreover, more contrast was used in all LRA procedures and in ACS patients, although there was no difference in mortality between the 2 approaches.
- -
Our results might be explained by the generally lower experience with the LRA in emergency scenarios.