Biomarkers, other than cardiac troponin, with potential sensitivity and selectivity that provide diagnostic and prognostic insights into the tissue–specific injury processes underlying acute coronary syndrome and their possible use in risk stratification algorithms are discussed. Such biomarkers may be useful as complementary or alternative to cardiac troponin (I or T) assays in early diagnosis of acute coronary syndrome, as well as for monitoring acute coronary syndrome progression and prognosis assessment. The information included in this article is based on a critical analysis of selected published biomedical literature accessible through the United States National Library of Medicine's MEDLINE-PubMed and Scopus search engines. The majority of articles cited in this review and perspective, except for a few historical publications as background, were published between January 2000 and December 2013.
Keywords
This literature review presents a critically selected set of publications from our comprehensive search of the published biomedical literature archived by the United States National Library of Medicine Library (MEDLINE-PubMed) and alternatively retrieved using the Scopus search engine. For this article, we selected original nonclinical and clinical research, and a representative sample of historical and recent reviews, that identify established and putative nontroponin biochemical markers (aka biomarkers) of acute coronary syndrome (ACS). This review identifies blood sample biomarkers in current use as complementary or alternative to high-sensitivity cardiac troponin (hs-cTn) assays and emerging tissue and ischemic process-related biomarkers proposed for use in ACS risk stratification. Clearly, risk stratification of patients screened for possible ACS is performed most effectively by combining patient history and available medical data with electrocardiographic evaluation and serial analysis of blood for relevant biomarkers; typically hs-cTn, myoglobin, and creatine kinase–MB fraction (CK-MB) assay results. This review includes both early-phase and late-phase nontroponin biomarkers purported to identify—with varying degrees of sensitivity and specificity to reliably detect underlying biological events and processes– ischemic injury to myocardial cells, endothelial cells, plaque formation and eruption, platelet aggregation and other blood cell reporters associated with clot formation, and coronary or myocardial inflammation.
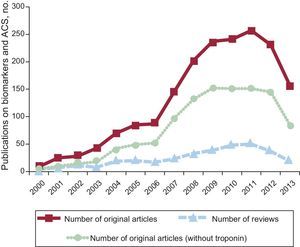
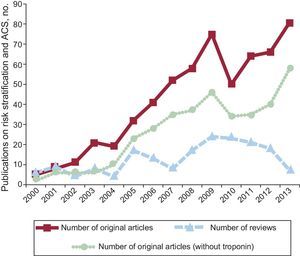
To set the stage, we compiled the frequency of original articles and reviews reporting on biomarkers and ACS (Figure 1) or on risk stratification and ACS (Figure 2), including original articles published for nontroponin biomarkers from January 2000 to December 2013 using the United States National Library of Medicine search engine, PubMed.1 While the number of original research publications reporting biomarkers for ACS appears to have reached an apex in 2011, publications related to risk stratification for ACS appear have steadily increased over the entire 14-year period, except for a small decline from 2009 to 2012. In a similar manner, the number of original research articles addressing ACS risk stratification with biomarkers other than troponin leveled out in 2009 at around 40 articles annually, with a slight increase in the number of original articles projected for 2013. The publication trends illustrated in Figures 1 and 2 were similar when we performed a literature search over the same time period with the same search terms, using the Scopus2 search engine. To assure that we had captured guidelines and consensus opinions and practices with ACS biomarkers, we also searched The Cochrane Library database.3
PubMed search results, 2000 to 2013 (2013 actual through October 31; estimated for 12 months), for the combined search terms “biomarker” and “acute coronary syndrome”. Total number of published original articles (solid line, squares), total articles without troponin as one of the biomarkers (dotted line, circles), and total number of review articles (dashed line, triangles). ACS: acute coronary syndrome.
PubMed search results, 2000-2013 (2013 actual through October 31; estimated for 12 months), for the search terms “risk stratification” and “acute coronary syndrome”. Total number of published original articles (solid line, squares), total articles without troponin as one of the biomarkers (dotted line, circles), and total number of review articles (dashed line, triangles). ACS: acute coronary syndrome.
The term ACS is used to describe myriad situations where the heart's coronary arterial blood supply is suddenly interrupted or blocked, typically as the terminal event of a progressive vascular disease process in one or more of the major branches of the coronary arterial circulation. ACS encompasses both classes of myocardial ischemia/infarction as assessed by electrocardiography —ST-segment elevation myocardial infarction and non—ST-segment elevation myocardial infarction— as well as unstable angina pectoris and coronary disease associated with various metabolic disorders (eg, diabetes mellitus).
The 2011 European Society of Cardiology Task Force defines ACS as “a life-threatening manifestation of [progressive] atherosclerosis […] usually precipitated by acute thrombosis induced by a ruptured or eroded atherosclerotic coronary plaque, with or without concomitant vasoconstriction, causing a sudden and critical reduction in [coronary/myocardial] blood flow.” The European Society of Cardiology, the American College of Cardiology Foundation, and the American Heart Association guidelines for ACS biomarkers place serial hs-cTn assays as the number one indicator, with CK mass, CK-MB isoenzyme, and myoglobin assays as secondary biomarkers.4,5 Clearly, early onset biomarkers that potentially identify cellular and tissue pathology events that underlie the progressive nature of ACS are receiving the most attention at present, as new diagnostic and prognostic screening tools and technologies are increasingly refined.
Whenever coronary blood flow supply providing nutrients and oxygen to myocardium falls below the required threshold to meet myocardial metabolic demand, a condition of relative myocardial ischemia presents and is associated with a switch from aerobic to anaerobic metabolism.6 When coronary flow reduction is sufficiently significant and sustained, myocardial tissue injury occurs. Due to physical forces during the cardiac cycle, the endocardial region of the ventricular wall is most susceptible to ischemia during such acute or progressive reductions in coronary perfusion pressure distal to points of stenosis or occlusion.
At the cellular level, regional myocardial ischemic injury leads to heart muscle cell membrane disruption and leakage of cell contents that may be detectable and useful as important biomarkers of ACS. A number of additional tissue type disruptions occur during ACS that involve pre-existing atherosclerotic plaques, endothelial cells, vascular smooth muscle cells, and blood elements including platelets, neutrophils and white blood cells.
CK was perhaps one of the earliest identified biochemical (intracellular enzyme) markers released into the blood following transient and sustained coronary artery occlusion.7–9 The myocardial isoenzyme CK-MB is perhaps the earliest reliable “gold standard” biomarker for assessment of ACS. In addition to CK-MB, assays for myoglobin and the cardiac contractile protein troponins (cTnI and cTnT) released from injured cardiac muscle cells are used in combination to diagnose or confirm ACS. Today hs-cTn assays are relied upon as the most relevant, sensitive, and specific overall reference biomarker for early and late-stage ACS and myocardial ischemic injury assessment in patients presenting at the emergency department after symptom onset.10 Clearly, the advent of hs-cTn assays, early-detection capability ACS has elevated the hs-cTn (I or T) as a biomarker of preference over earlier candidates, including myoglobin and copeptin.11
Current assessments to confirm ACS include blood biomarker tests for cardiac proteins and enzymes, typically cardiac troponin I and T (hs-cTnI, hs-cTnT), CK-MB, myoglobin, C-reactive protein (CRP), fibrinogen, homocysteine, lipoproteins, triglycerides, brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) and prothrombin, etc. In contrast, biomarkers for congestive heart failure, in addition to high-sensitivity assays for cardiac troponins, often include BNP or NT-proBNP and galectin-3.
Such ACS serum biomarker tests, typically used in conjunction with electrocardiogram analysis to determine that myocardial ischemia exists, can be useful in estimating the extent of myocardium that has been damaged by a coronary flow deficit. Each biomarker has a unique temporal profile in serial blood samples.12 Some biomarkers, such as myoglobin, may be elevated early and return to baseline within 4 h to 6 h after event onset, while others, such as troponin, increase more slowly in the blood and may sustained for days after the onset of the coronary perfusion defect precipitating ACS. Additional ACS-associated biomarkers—such as beta-trace protein, cystatin C, glycogen phosphorylase isoenzyme BB, mid-regional pro-adrenomedullin and mid-regional pro-atrial natriuretic peptide—exhibiting varying degrees of sensitivity and specificity for the underlying tissue and cellular events continue to be identified. Patients presenting with the spectrum of symptoms and history consistent with ACS, particularly those presenting with unstable angina or non—ST-segment elevation myocardial infarction, are more likely to be correctly diagnosed with ACS if independent and complementary biomarkers are considered in the diagnosis and prognosis.13 Patients presenting with unstable angina and/or non—ST-segment elevation myocardial infarction also may display sex-specific cardiac biomarker patterns. Men were more likely to have elevated CK-MB and troponins, whereas women were more likely to have elevated CRP and BNP.14 This study suggests that more patients, especially more women, were correctly diagnosed with ACS when multiple biomarkers were considered.
The current recommendation for diagnosis of ACS is established by a joint task force of the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Health Foundation, together with the National Academy of Clinical Biochemistry. These organizations have recommended an elevation of 3 standard deviations (∼20%) in hs-cTn levels as a requirement for confirmation of myocardial infarction in ACS.
Biomarker levels and their temporal profiles can also be useful in assessing the future risk of coronary artery disease, morbidity, and mortality. Exercise stress tests using standard protocols while monitoring blood pressure and electrocardiographic changes (eg, ST segment elevation) are certainly useful in coronary artery syndrome prognoses. Additional approaches, beyond blood biomarkers, used to confirm the location and extent of myocardial injury in ACS include coronary angiography to visualize obstructions in coronary arteries, cardiac radionuclide perfusion imaging to identify coronary flow deficits at rest and during pharmacological challenge tests,15 echocardiography to determine overall left ventricular function and assess myocardial regions exhibiting systolic dysfunction, magnetic resonance imaging, and positron emission tomography scanning. Beyond these methods, functional flow reserve evaluation is advocated to assess the dynamic range of coronary flow and myocardial viability.16,17
Beyond the more conventional and generally accepted array of ACS biomarkers noted above are a number of new candidates arising from laboratory and clinical studies in the wake of recent advances in biotechnology and cell biology. These new markers include cell injury biomarkers—such as micro-RNAs (miRNAs), circulating cell-free DNA, circulating microparticles, microvesicles (MVs), exosomes—, and selective biomarkers for endothelium, atherosclerotic plaques, vascular smooth muscle and myocardial cells injury. Circulating cardio-enriched miRNAs18 and cell-free circulating DNA19 report myocardial ischemic events. Circulating microparticles and MVs may also be useful ACS biomarkers.20 Novel biomarkers of endothelial dysfunction or disruption include soluble amyloid β precursor protein 77021 and serum pigment epithelium-derived factor.22 Fibroblast activation protein-α is a membrane glycoprotein expressed in and released from atherosclerotic plaques.23 High-sensitivity CRP, paraoxonase-1, secretory phospholipase A2, and various myeloperoxidases (MPOs) and metalloproteinases are also proposed as useful ACS biomarkers.24 Cathepsin-K, a potent circulating collagenase, is also proposed as a sensitive ACS biomarker.25
There is no doubt that the development of hs-cTnI or hs-cTnT assays has improved the diagnostic rate and accuracy of ACS, but there remains a subset of patients that present with apparent ACS and normal troponin levels.26 This group of patients has a significant risk of myocardial infarction within 1 year of follow-up,27 thus demonstrating the need for sensitive and specific nontroponin biomarkers for ACS. In addition, hs-cTn assays carry a significant risk of false positive reporting.28 A recent survey by Yayan29 recently identified, in addition to the established biomarker CRP, 46 novel inflammation biomarkers associated with coronary artery disease. Additional time and scrutiny will reveal which, if any, of these new biomarkers are sufficiently sensitive and specific for use in ACS diagnosis and risk stratification.
BIOMARKER SPECIFICITY AND SENSITIVITYOne typically considers the terms “sensitivity” and “specificity” in the context of the assay method applied to detect a biomarker. While methods and techniques to detect specific biomarkers are continually refined to increase the lower levels of detection (“sensitivity of measurement”) and to measure only a singular biomarker of interest as separable from other possible analytes in a sample (“selectivity of measurement”), some methods such as mass spectrometry and nuclear magnetic resonance spectroscopy are capable of detecting multiple molecular entities that as a collection may offer advantages as both sensitive and specific biomarker arrays, compared to a single biomarker.30
Certainly, biomarkers should be “specific for the process”, disease entity, or stage of disease being probed and demonstrate sensitivity not only in the method of assay (eg, antibodies that target a specific and stable epitope on troponin I), but perhaps more importantly “sensitivity in proportion to the magnitude of the process” for which the biomarker has demonstrated specificity. For example, if a biomarker –for example, BNP- arises predominantly from the cardiac atria and such BNP release into the bloodstream is related to the magnitude of atrial stretch caused by blood volume expansion related to the various progressive stages of heart failure, then BNP would demonstrate both specificity and sensitivity of clinical value. In a similar manner, if a marker of atherosclerosis was measured, and the atherosclerosis was systemic, then the marker's relationship to coronary artery atherosclerosis might be neither specific nor sensitive to that target site of the disease, and therefore might be of limited value in assessing this component of coronary artery syndromes. In yet another example, if a left ventricular region is injured due to coronary blood flow reduction or obstruction, then a biomarker of this injury would be useful, as would a biomarker that was both specific to the ischemic injury and to the magnitude of the ischemic myocardium.
Inflammation is yet a fourth case where coronary artery inflammation would be of interest, and the biomarker sought should be not only a sensitive reporter of a particular type of inflammatory process, but also a specific reporter of only the inflammatory process in the coronary circulation. Perhaps the best, but not always practical, way to improve biomarker specificity would be to perform sampling or detection at the specific source of the disease of interest. This might be achievable by labeling and imaging a particular biomarker at the site of its generation or release, and in a more targeted way by obtaining blood samples from supply arteries and draining veins from the tissue or organ of interest, thereby improving tissue (ie, locus) specificity. When ACS is the disease of interest, blood samples simultaneously drawn from a systemic artery and from the coronary sinus would identify biomarkers for cardiac-specific events.31
Biomarker signal dilution is a serious challenge when relying on peripheral blood samples to detect biomarkers specific for and sensitive to ACS, and as we previously suggested32 and others30 have proposed, a single biomarker may be neither as specific nor as sensitive in ACS prognosis, extent, or progress as would be a set of biomarkers that taken together increase both sensitivity and specificity for assessing the syndrome and perhaps particular events within the syndrome. This combinatorial approach is taken when hs-cTn, myoglobin and CK-MB data are combined to improve ACS diagnostic and risk assessment reliability.33–39
MYOCARDIAL CELL ACUTE CORONARY SYNDROME BIOMARKERSBiomarkers that are passively released from cardiac myocytes consequent to ischemic injury are the most commonly relied-upon indicators of ACS and subsequent cell injury. Myoglobin, CK-MB and cardiac troponins are the current conventional biomarkers in this category. In addition, if the mass of left ventricle affected by the coronary insufficiency is large enough to increase wall stress (dilate the region during diastole and produce paradoxical bulging during systole, then the stretched cardiac myocytes can and will release BNP and NT-proBNP.28 Other factors that are released from damaged or dying myocardium include CRP, BNP, ischemic modified albumin, and heart-type fatty acid binding protein.35,40–42
Over the years, a number of previously used biomarkers of ACS —aspartate aminotransaminase, total lactate dehydrogenase, and lactate dehydrogenase isoenzymes—have been abandoned due to poor cardiac injury specificity and because of their ubiquitous tissue distribution. Early reports identified other cytoplasmic molecules released from transiently or permanently injured myocardial cells. Among these biomarkers was CK and the subsequent identification of myocardial and brain isoforms.7–9 At present, the hs-cTn assay, based on its superior tissue specificity and improved sensitivity to low cardiac troponins levels in blood samples, is the standard against which other putative ACS biomarkers are evaluated. The 99th percentile (3 standard deviations above the normal average value) is used as the decision limit for confirmation of myocardial injury by hs-cTnT, CK-MB mass, and myoglobin.
In addition to the release of cytoplasmic enzymes or protein products into the blood stream, damaged cells may also release nucleic acids that can be specifically detected as biomarkers. Several recent studies have shown increased serum levels of cell-free circulating DNA following cardiac ischemia, presumably consequent to myocyte apoptosis and necrosis.43–45 Increased serum levels of cell-free circulating DNA have been reported in ACS patients and were further elevated following ST-segment elevation myocardial infarction.19,46 However, the utility of cell-free circulating DNA as a biomarker may be limited by its relatively short half-life < 30 min.47 Conversely, while a short half-life may limit its utility in determining the degree of acute ischemic damage, its rapid clearance from circulation may render it beneficial in differentiating between discrete and sustained ischemic events if monitoring and blood sampling occurs proximate to coronary events.
INFLAMMATION BIOMARKERS IN ACUTE CORONARY SYNDROMEThe previous section described the secretion (passive or active) of biomarkers from injured myocardium following the initiation of an ischemic event. In contrast to these biomarkers, which are indicative of a prior or ongoing ischemia, the biomarkers discussed in the next two sections (markers of coronary inflammation or atherosclerosis) are more predictive because they are often present prior to the ischemic damage or myocardial necrosis/apoptosis. In addition, coronary inflammation is a primary driving force for the development and progression of atherosclerosis and, thus, increased inflammation is also a common indicator of coronary atherosclerosis.
Among mediators and markers of coronary and myocardial inflammation are a number of acute-phase proteins, cytokines, interleukins (IL), and cell adhesion molecules, the foremost of these being CRP. Inflammation of and other injury to the vascular endothelium is considered the cardinal event leading, over time, to the establishment of atherogenic plaque, which may serve as the focal point for eventual platelet aggregation and thrombosis and/or eruption of the plaque's protective fibrous cap, for which CRP, as well as IL-6 and serum amyloid A, is used as the leading biomarker within the ACS paradigm. The release of CRP, detected by a high-sensitivity assay (high-sensitivity CRP), can also produce secondary effects such as the expression of adhesion molecules, among other events which themselves may be biomarkers associated with ACS. A decade ago, a number of other putative biomarkers for ACS risk stratification were emerging. These included markers of platelet activation, enzymes such as metalloproteinases that disrupt an atheroma's fibrous cap integrity, and MPOs released from leukocytes activated within the coronary circulation.
The most common and widely used marker of coronary inflammation that predicts myocardial ischemia risk is CRP.48–52 However, other inflammatory biomarkers, including pentraxin 3, serum amyloid A, fibrinogen, and MPO, have proven to be equally or more predictive for ACS and subsequent cardiac events. Pentraxin 3, an early-phase ACS response protein that is a more specific marker of vascular inflammation than is CRP, is released by multiple cell types, including fibroblasts and dendritic and endothelial cells, in response to primary inflammatory signals.53,54 Serum amyloid A and fibrinogen are also early-phase ACS response proteins whose serum levels are elevated in response to acute inflammation.55 Neutrophil cell count and activation are also markers of inflammation, and levels of the neutrophil-derived lysosomal enzyme MPO can be a sensitive biomarker for inflammation and cardiovascular risk.
First identified in 2003 as a predictive biomarker for ACS in the absence of myocardial necrosis, MPO can serve as a marker for coronary inflammation and cardiac risk much earlier than myocardial ischemia and infarction biomarkers—myoglobin, troponin, CK-MB, or CRP—in patients evaluated soon after onset of symptoms.56,57 Recent studies have confirmed the utility of MPO as a biomarker of coronary inflammation and shown that its long-term predictive power is further increased when combined with a high-sensitivity CRP assay.48,58
Additional biomarkers have been investigated as potential markers of coronary inflammation and ACS, but their clinical usefulness as sensitive and specific predictive biomarkers has not yet been determined conclusively. Examples of these potential biomarkers include soluble CD40 ligand, tumor necrosis factor-α, IL-10, and IL-18. Both soluble CD40 ligand and tumor necrosis factor-α are known initiators of pro-inflammatory signaling and are elevated in patients with ACS. However, none of these appears to be a specific predictor of ACS or of subsequent adverse cardiac events.59,60 Although IL-18 is a pro-inflammatory cytokine that is thought to initiate plaque instability and is elevated in association with other established risk factors, it does not independently predict ACS or myocardial infarction risk.61
BIOMARKERS OF CORONARY ATHEROSCLEROSISThe progressive precedent event and process leading to ACS is coronary arterial atherosclerosis. As such, selective biomarkers can allow detection of rapidly changing events related to atherosclerotic plaque, including the rupture of its protective fibrous cap.
BIOMARKERS ARISING FROM DAMAGED CORONARY ENDOTHELIUMIntegrity of the coronary endothelium is necessary for capillary homeostasis and for proper physiological functioning of the underlying vascular smooth muscle of the coronary arterial segments leading to the capillaries.62 The endothelium is the blood interface and the first barrier and transducer of the vasomotor effects of microfluidic shear force changes and of certain vasoactive agents working through nitric oxide. Injury to or inflammation of the coronary endothelium alters these barrier functions, and may lead to the presence of biomarkers and MVs in the coronary circulation that can be detected in system serum samples.63–66
BIOMARKERS OF PLATELET ACTIVATION AS PRECURSOR TO CORONARY ARTERIAL THROMBOSISPlatelet aggregation can occur subsequent to activation of the fibrinogen receptor, glycoprotein IIb/IIIa, and the endothelial cell adhesion molecule PECAM-1. Once activated, biomarkers may be shed by platelets as free proteins, miRNAs, or MVs.
CARDIAC-DERIVED MICRO-RNAS AS ACUTE CORONARY SYNDROME BIOMARKERSMiRNAs are an emerging class of biomarkers that are likely to be actively released by cardiac cells in response to ischemic damage. Changes in miRNA expression within myocytes have been known for some time to vary in response to ischemia, and evidence is building that the expression profile of cardiac-derived circulating miRNAs represents the environment and conditions of the myocardium. Circulating miRNAs have advantages as biomarkers in that they have a favorable expression time-course (rapid onset and long-lasting), are stable in circulation and post-isolation, and can be very specifically detected at very low levels.25 The studies that have thus far investigated circulating miRNAs as biomarkers for ACS and myocardial ischemia/reperfussion injury were recently reviewed by Deddens et al.67 Another appeal of miRNAs as ACS biomarkers is their tissue-specific expression. For example, miR-208 is expressed in a cardiomyocyte-specific manner from an intron in the alpha-MHC gene. Several studies have taken advantage of this to investigate the secretion of miR-208 as a specific marker for cardiomyocyte ischemic injury. While not all reports regarding miR-208 as a specific biomarker for ACS are positive, multiple reports show a rapid increase in circulating miR-208 post-myocardial infarction.68–72 MiR-499 is another miRNA specific to cardiac muscle that has shown promise as an early marker of myocardial infarction.68,73
Perhaps the most promising circulating miRNA biomarker is miR-1, a muscle-specific biomarker shown by numerous studies to be elevated following both ST-segment elevation myocardial infarction and non—ST-segment elevation myocardial infarction cardiac ischemia.72 In addition to muscle-specific miRNAs such as miR-1, miR-208, and miR-499, miRNAs from other tissue types such as vascular smooth muscle, endothelium, and leukocytes also represent potential biomarkers of components of ACS. The investigation of miRNAs as biomarkers is still novel, with the majority of the publications coming in just the last 3 to 4 years. New miRNAs are still being identified and their use as biomarkers has substantial potential.
CARDIAC–DERIVED MICROVESICLES AS ACUTE CORONARY SYNDROME BIOMARKERSSome of the biomarkers discussed above are not simply released passively as a result of cellular injury, but rather are actively released as a specific, dynamic, cellular response to ischemic stress. Numerous reports have described the release of extracellular MVs from cardiomyocytes, endothelial cells, and neutrophils. Such MVs may play an active role in the development or progression of ACS.65,66,74 The content and release of these MVs appear to be pathology-dependent, further suggesting that they may serve a specific role in signaling to distant target tissues/cells.64,66,75,76 Secreted MVs are known to contain defined protein components, and at least 3 of these (polygenic immunoglobulin receptor, cystatin C, and complement factor C5a) may also serve as useful ACS biomarkers.77
In addition to component proteins, other studies have directly measured MVs and the cell types from which they originate and have implicated endothelial cell-derived and monocyte-derived MVs as independent predictors of myocardial damage in non—ST-segment elevation myocardial infarction patients.20 Circulating MVs may represent an entire unique class of distinguishable biomarkers that can be utilized in combination with each other depending on tissue source or content. This is exemplified by studies that examined multiple distinct MVs populations or the ratios of these populations as independent biomarkers.20
Recent discovery of exosomes containing molecules that report cell-specific information and participate in intercellular communication signaling and delivery of encapsulated materials from one cell to another suggests that the search for ACS biomarkers will continue to evolve. In the near future, the analysis of cardiac-origin exosome contents and the interpretation of this content profile may reveal yet another avenue by which early ACS diagnosis, prognosis, and risk stratification will be refined.
NOVEL GENE AND PROTEIN BIOMARKERS FOR ACUTE CORONARY SYNDROME DIAGNOSIS AND RISK STRATIFICATIONIn a recent report by Silbiger et al,78 whole genomic expression analysis using expressed mRNA profiles on gene chip microarrays detected 549 differentially expressed genes in peripheral blood of ACS patients within the first 48h. Within these hundreds of genes expressed during the early onset of ACS, 13 genes were considered both technically and biologically validated by real time-PCR, and all were statistically expressed differentially in ACS patients in comparison with control patients in both phases of the study. The authors suggest that changes in ALOX15, CA1, and KCNE1 genes may reflect a “protective system response” following coronary occlusion, and that BCL2A and COX7B genes may report apoptosis regulation in endothelial cells and cardiac myocytes. The authors further suggest that 6 genes (AREG, IL18R1, IRS2, MYL4, BCL2L1, and MMP9) may report endothelial and cardiac tissue remodeling after ischemia, and that other combinations among these 13 genes may indicate atherosclerotic plaque progression and rupture, or may serve as triggers in the inflammatory cascade.
In a similar manner, the IBIS-1 pilot study by Wykrzykowska et al79 applied microarray technology to correlate coronary imaging (intracoronary ultrasound imaging and multi-slice computed tomography scanning) with circulating biomarker expression in patients with the whole spectrum of coronary syndromes. Elevations were detected in high-sensitivity CRP, IL-6, lipoprotein-associated phospholipase A2 activity, and NT-proBNP, but not in tumor necrosis factor-α or soluble CD40 ligand levels. Anti-apoptotic markers (eg, plasminogen activator inhibitor type 1) increased over time. Pro-inflammatory markers and markers of lymphocyte trafficking (eg, C-6 Kine, CTAK) increased initially and then decreased over time, as did markers of coagulation (eg, D-dimer) and of endothelial shear stress and remodeling (eg, follistatin).
Such gene expression and transcriptional profiling results suggest that future studies will identify and refine a critical subset of gene products as biomarkers for use throughout the ACS time-course for improved patient risk assessment and stratification. Clearly, future efforts will be directed at refining both sensitivity and specificity of these gene biomarkers to gain prognostic value in ACS and at identifying which gene biomarkers shed light on tissue-specific events underlying ACS.
MATRIX METABOLOMICS STRATEGIES FOR ACUTE CORONARY SYNDROME DIAGNOSIS AND RISK STRATIFICATIONIn recognition that a single biomarker may be insufficiently sensitive or specific for risk stratification of ACS patients, Bodi et al.30,80 reported recently on an improved approach for ACS risk assessment using multiple biochemical markers. It is reasonable to forecast that by applying contemporary analytic methodologies such as high-throughput magnetic resonance spectroscopy and mass spectrometry, patient blood samples may be analyzed for ACS-related changes using computational algorithms that stratify patient ACS risk.32 Risk assessment, including risk calculators, of lipids and cardiovascular disease are available throughout much of Europe and promulgated by British82–84 and Spanish85,86 cardiology and atherosclerosis professionals. While the search for algorithms and calculators that integrate biomarker values to assist predictive risk stratification is laudable, unreliable outcomes may result when outdated biometrics are used to create the calculation that indicates a need for cholesterol-lowering drugs (statins). Specifically, the use of the new guidelines and risk calculator recently endorsed and published jointly by the American Heart Association and the American College of Cardiology81 apparently leads to a substantial overestimation of risk and potential over-prescribing of this class of medicines to individual patients who may not truly be at elevated risk for atherosclerosis, which is one component of ACS.
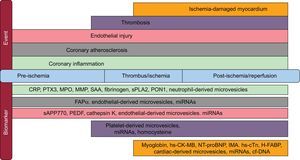
THE FUTURE OF ACUTE CORONARY SYNDROME BIOMARKERS AS AIDS TO EARLY DIAGNOSIS, PROGNOSIS, AND RISK STRATIFICATIONSensitive, Cell/Process Specific, and Temporally Correlated BiomarkersThe priorities for biomarker use in ACS risk assessment and stratification require further work to characterize not only the temporal correlation of each biomarker or assembly of biomarkers to each of the progressive stages of ACS (Figure 3), but also an objective assessment and verification of each biomarker's sensitivity and specificity to an underlying pathology component contributing to ACS itself. The selection of appropriately sensitive biomarkers that identify underlying tissue-specific and cell-type-specific changes in a manner that aligns the temporal pathology events referenced to onset of ACS symptoms will be the most useful for accurate diagnosis and risk stratification of such patients. It is our opinion that the rapidly expanding wealth of information arising from research in nonclinical animal models of ACS and from clinical studies in ACS patients will soon, after substantial effort, allow the possibility of selecting the most specific and sensitive biomarkers for each of the underlying features that present as ACS.
Acute coronary syndrome biomarkers associated with the underlying myocardial events. cf-DNA, cell-free circulating DNA; CRP, C-reactive protein; FAP-α, fibroblast activation protein-α; H-FABP, heart-type fatty acid binding protein; hs-CK-MB, high sensitivity-creatine kinase–MB fraction; hs-cTn, high sensitivity-cardiac troponin; IMA, ischemic modified albumin; miRNAs, micro RNAs; MMP, metalloproteinases; MPO, myeloperoxidases; NT-proBNP, N-terminal pro-brain natriuretic peptide; PEDF, serum pigment epithelium-derived factor; PON1, paraoxonase-1; PTX3, pentraxin 3; SAA, serum amyloid A; sPLA2, secretory phospholipase A2; sAPP770, soluble amyloid β precursor protein 770.
Efforts must not only continue to explore and apply new analytic technologies to identify putative ACS biomarkers, but must also seek to understand the link to the most specific and sensitive of myriad biomarkers, not simply as correlations to early- or late-stage ACS, but to each of the underlying biological processes, including the status of coronary atherosclerosis plaques, evidence of platelet aggregation and clot formation, smooth muscle and endothelial cell health, cardiac myocyte integrity, and perhaps even myocardial sympathetic innervation status.87
The speed and reliability (sensitivity and specificity) of biomarker test reporting for patient diagnosis and prognosis, risk stratification, and therapeutic decision-making is increasingly a conversation connecting point-of-care testing with emerging technologies and devices, as contrasted to conventional central laboratory testing methods and facilities. Most recommended ACS biomarkers (ie, CK-MB, myoglobin, BNP and/or NT-proBNP, and possibly excepting hs-cTnI and/or hs-cTnT) can be elevated in conditions other than ACS (eg, hypertension, skeletal muscle trauma, renal failure, primary aldosteronism, and thyroid disease). Therefore, the search continues for unique individual or sets of biomarkers that are both sensitive and specific for each of the underlying pathologies and processes that comprise ACS onset and progression, as well as those biomarkers that will be most useful and effective for ACS risk stratification and optimal therapeutic decisions.
Trends Toward Point-of-care Multiple Biomarker AssaysAmong the currently identified biomarkers, there is certainly a potential for redundancy in reporting information related to any of the underlying biological events. At some near future date, it is likely that point-of-care, microchip-array, rapid-response assay systems will integrate the combination of serum protein and gene biomarkers, biochemical enzymes, and cell membrane markers to permit a comprehensive and detailed decision-directed diagnosis of ACS and guide improved risk stratification of ACS patients.88 The current conversation regarding conventional and novel or emerging ACS biomarkers—beyond considerations of laboratory method and pathology specificity and sensitivity—includes the topic of laboratory test turnaround time and the cost/benefit of decreasing it with reliable and reproducible testing at the site of patient evaluation. Point-of-care testing by primary care physicians using a 3-in-1 device capable of rapidly reporting ACS, heart failure, and thromboembolism biomarkers (cTnT, NT-proBNP, or D-dimer, an indicator of fibrin degradation and coagulation activation) has been reported to improve differential diagnosis of potential ACS during out-of-hospital visits.89
The value proposition regarding emerging and novel biomarkers continues to consider whether adding one or more biomarkers (multi-marker approaches) will improve risk stratification and prognostic advantage over current and more conventional stratification strategies.12,90 On this point, an incremental ACS prognostic benefit of combining NT-proBNP and growth differentiation factor 15 with the GRACE score plus a corrected hs-cTnT value has been demonstrated, considering a combined endpoint of 6-month all-cause mortality or nonfatal myocardial infarction.91 By comparison, the 1-year predictive value of a number of inflammatory and noninflammatory markers assessed in ACS patients in the SIESTA study showed that only NT-proBNP and fibrinogen were sensitive and specific prognostic biomarkers of cardiovascular risk (all-cause mortality at 1-year follow up).92 In this study, few if any of the 10 examined biomarkers (eg, cystatin, CRP, E-selectin) added information to that provided by conventional clinical risk markers; however, more than three fourths of the patients in this study were receiving statin (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) treatment known to exert anti-inflammatory actions, a potential confounding variable in the study. Such approaches as those employing point-of-care assays and lab-on-a-chip microarrays linked to predictive diagnostic, prognostic, and risk stratification algorithms will potentially reduce false positive ACS diagnoses and unwarranted hospitalizations, and result in health care savings and improvements in the informed use of therapeutics to the benefit of patient health.
CONFLICTS OF INTERESTNone declared.
The authors wish to thank Ms. Kristin Luther for her assistance in creating Figure 3.
Section sponsored by AstraZeneca