Tricuspid valve (TV) dysfunction is common in patients with other diseased valves and heart conditions. Tricuspid regurgitation (TR) is often found in patients with left-sided valve disease, especially when there is pulmonary hypertension. Over 30% of candidates for mitral valve surgery have moderate or severe TR. In most cases there is a functional component, due to the remodeling of the right ventricle (RV). More rarely, the valve itself is affected by different processes that alter its morphology and hinder its function. Although TR can be well tolerated for years, it has a clear clinical impact. Moderate or severe TR in patients with left-sided valve disease reduces survival, limits exercise capacity, and impairs functional status.
Despite the high prevalence of this valve disease, TR is an uncommon topic in the medical literature in Spain. In an article published in this issue Revista Española de Cardiología, Rodríguez-Capitán et al.1 have presented their findings after treating this valve disease for more than a decade. The authors analyzed the epidemiology of severe TR and the clinical and functional outcomes of surgery, correlating them with the type of surgical procedure.
FUNCTIONAL TRICUSPID REGURGITATIONIn most patients, TR has a strictly functional mechanism, which is the result of ventricular disease. The TV should be systematically assessed in all patients scheduled to undergo valve surgery, with performance of an echocardiographic study to identify TV morphology and function, as well as RV dimensions and systolic function. As is often the case in Spain, the series of Rodríguez-Capital et al.1 did not analyze the impact of RV dimensions and function on the clinical and functional results of surgery, probably because no data were available for these factors. The systematic assessment of the RV in valve disease is still a pending issue in contemporary cardiology.
Indications for Surgical InterventionThe indication for surgical intervention in functional TR usually depends on whether the associated left-sided valve disease requires intervention. In the past, surgeons have shown a fairly conservative approach to TR. Surgical correction was considered unnecessary when TR was mild or moderate, or only detected intermittently. It was presumed that, in these cases, the TR would resolve after the left-sided disease was corrected, especially if there was no right chamber dilatation or elevated pulmonary pressure. In line with this school of thought, Rodríguez-Capitán et al.1 considered surgical interventions only for severe and symptomatic TR. However, it has become increasingly apparent that many patients who do not undergo surgical intervention have persistent TR that progresses over time and becomes the main cause of functional limitation. Up to 50% of TR cases that are not corrected at the time of surgery are severe after 5 years.2
Today there is evidence-beit not irrefutable-that supports a more aggressive approach to TR. Those who are strong advocates of intervention even defend prophylactic tricuspid annuloplasty (TA) in the absence of marked TR.3 The aim is to prevent progressive dilatation of the tricuspid annulus, which invariably leads to severe TR. These proponents justify their approach by citing the simplicity of the technique, the fact that it does not increase surgical risk, and the high risk entailed in reoperations due to residual TR. Recent studies have shown that when the tricuspid annulus is already dilated, correcting mild or moderate TR at the time of surgery avoids medium- and long-term progression.4
Years ago, surgical correction of isolated functional TR was highly unusual, but this is no longer the case. This type of TR is usually seen in patients who did not undergo correction of TR during a previous intervention, or whose TR was insufficiently corrected. In these patients, tricuspid and/or RV dysfunction causes persistent disabling symptoms that respond poorly to conservative treatment. Unfortunately, in many of these patients, RV function has deteriorated to such a degree that it does not improve with valve repair, and can actually worsen.
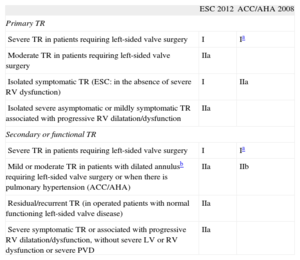
The recommendations in the main guidelines for the management of valvular heart disease, published by the European Society of Cardiology and American College of Cardiology/American Heart Association,5,6 are shown in Table 1. These recommendations are not is based on evidence from scientifically robust studies, but on extrapolations of observational studies or expert opinion.
Indications for Tricuspid Regurgitation Surgery
| ESC 2012 | ACC/AHA 2008 | |
| Primary TR | ||
| Severe TR in patients requiring left-sided valve surgery | I | Ia |
| Moderate TR in patients requiring left-sided valve surgery | IIa | |
| Isolated symptomatic TR (ESC: in the absence of severe RV dysfunction) | I | IIa |
| Isolated severe asymptomatic or mildly symptomatic TR associated with progressive RV dilatation/dysfunction | IIa | |
| Secondary or functional TR | ||
| Severe TR in patients requiring left-sided valve surgery | I | Ia |
| Mild or moderate TR in patients with dilated annulusb requiring left-sided valve surgery or when there is pulmonary hypertension (ACC/AHA) | IIa | IIb |
| Residual/recurrent TR (in operated patients with normal functioning left-sided valve disease) | IIa | |
| Severe symptomatic TR or associated with progressive RV dilatation/dysfunction, without severe LV or RV dysfunction or severe PVD | IIa | |
ACC/AHA, American College of Cardiology/American Heart Association; ESC, European Society of Cardiology; PVD, pulmonary vascular disease; TR, tricuspid regurgitation; RV, right ventricle; LV, left ventricle.
Correction of functional TR is mainly focused on reducing annular dilatation. The various techniques used in TA aim to reshape the valve annulus and thus increase the contact surface of the leaflets during systole. This reshaping results in valve competence without compromising ventricular filling. It can be achieved by suturing folds in the tricuspid annulus or by implanting a ring and attaching it to the annulus, thus reshaping the annulus and reducing its diameter.
Of the many techniques that have been developed, the De Vega repair7 -with its different variants-has become the most popular, and is the technique preferentially used by Rodríguez-Capitán et al.1 This technique reduces the annulus corresponding to the anterior and posterior leaflets with a double suture that can be brought out, if necessary, to adjust the diameter in a controlled manner. With the original technique, the ring is plicated mainly at the expense of the posterior segment; the suture is tied at the end of this segment. Although in theory this technique preserves the movement of the tricuspid annulus, in most patients with functional TR, such preservation is irrelevant in practice because the tricuspid annulus has already become deformed and has lost its normal movement.
Although this technique is a simple and inexpensive, the outcome is less reproducible and, above all, less stable than that of ring annuloplasty. Most studies that have compared the 2 techniques have found a higher rate of TR recurrence and a more frequentneed for reoperation with the De Vega technique.8
In ring TA, the reducing effect is achieved by tying multiple suture knots to attach the prosthetic ring to the anatomical annulus. The necessary reduction in valve orifice has no significant hemodynamic consequences, although it is common to detect a certain effect on the transvalvular gradient, especially in small rings. Since there are different sized rings, surgeons are able to control the amount of tricuspid annulus reduction. Furthermore, the ring itself remodels the native annulus to a more anatomical shape and provides support to sustain the reduction. There is a wide variety of rings for performing tricuspid repair: rigid, semi-rigid and flexible, complete and incomplete, and 2- and 3-dimensional versions. This diversity allows surgeons to choose the most appropriate ring for each patient but also reflects the lack of consensus on the ideal characteristics for a ring.
The aim of rigid or semi-rigid rings is to remodel the dilated tricuspid annulus, returning it to its anatomical shape by restoring the anatomical proportions of the different segments. Loss of systolic contraction, which is already absent in most patients with severe TR, is not a problem; restoration of systolic contraction does not improve valve competence if such competence has already been achieved by reducing the valve orifice. The latest rigid rings have a 3-dimensional design that imitates the anatomical shape of the tricuspid annulus, thus aiding implantation and reducing the risk of dehiscence.
Flexible rings aim to preserve the normal movement of the valvular plane, although the majority of patients with severe TR no longer have such movement. This type of ring has not been shown to afford better valve competence or to preserve ventricular function more effectively than rigid rings.
Most comparative studies have found that rigid or semi-rigid rings provide the best assurance of stable repair in the long-term.9 Although only a few rings were used in the series by Rodríguez-Capitán et al.,1 their results also corroborate this finding.
When there is significant ventricular dilatation and marked leaflet tenting, complementary procedures may be necessary to guarantee valve coaptation. One approach is an edge-to-edge approximation of the middle points of the free margins of the leaflets (clover technique), using a similar technique to that used in the mitral valve.10 Although its creators have reported low rates of TR recurrence in the medium-term in patients with complex tricuspid disease, this technique is more of a complementary procedure than an alternative to TA. Dreyfus has proposed a more physiological technique, albeit more aggressive technically, consisting of augmenting the anterior leaflet surface by applying a large pericardial patch.3 This technique compensates the apical movement of the papillary muscles, reducing leaflet tension during systole and increasing the coaptation surface, but it also needs to be complemented with an annuloplasty. However, the long-term outcome of these 2 techniques is still unknown. If neither of these procedures is sufficient, valve replacement with full preservation of the subvalvular apparatus may be the only option for correcting TR.
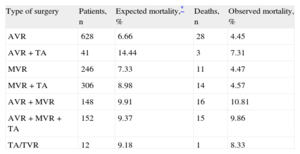
Risks and Outcomes of Tricuspid CorrectionAn intervention on the TV during a polyvalvular procedure does not significantly increase surgical risk. Most studies have found that TA does not increase the risk of mortality or complications beyond those inherent to left-sided valve disease correction. In our experience (unpublished data), the association of TA with mitral or mitral-aortic correction did not increase early mortality (Table 2). Interestingly, TA was, however, found to increase early mortality in patients with isolated aortic valve disease, a group with a higher risk profile. Although the study by Rodríguez-Capitán et al. provides no data on this association, the operative mortality (18.5%) was not surprising, considering the population under study, although it was higher than the 12.3% estimated by the logistic EuroSCORE.
Effect of Tricuspid Repair Associated With Left-sided Valve Surgery on Mortality
| Type of surgery | Patients, n | Expected mortality,* % | Deaths, n | Observed mortality, % |
| AVR | 628 | 6.66 | 28 | 4.45 |
| AVR+TA | 41 | 14.44 | 3 | 7.31 |
| MVR | 246 | 7.33 | 11 | 4.47 |
| MVR+TA | 306 | 8.98 | 14 | 4.57 |
| AVR+MVR | 148 | 9.91 | 16 | 10.81 |
| AVR+MVR+TA | 152 | 9.37 | 15 | 9.86 |
| TA/TVR | 12 | 9.18 | 1 | 8.33 |
AVR, aortic valve replacement; MVR, mitral valve replacement; TA, tricuspid annuloplasty; TVR, tricuspid valve replacement.
The data refer to 1533 patients undergoing isolated valve surgery in the University Hospital of Salamanca.
The outcome of TV surgery is less predictable than that of other valves; outcome is often suboptimal because of the complex anatomy of this valve and delayed indication for correction. However, TA restores valve competence in most patients and improves their quality of life and survival in the medium- and long-term. Nevertheless, as many as 40% of patients who have undergone TA are found to have severe TR at 10 years.9 The probability of residual TR is associated with the degree of TR, tricuspid annulus dimensions, tenting height and area, RV dimensions and morphology, and repair performed without a prosthetic ring.
Reoperation to repair residual/recurrent TR carries a high mortality risk of between 20% and 40% and poor long-term survival.11 Moreover, in this setting, TR correction is often unable to improve the poor natural history of this disease, and a significant proportion of patients show no improvement in functional class after surgery. The only option to improve the prognosis of these patients is early reoperation, even if they have an acceptable functional status, and especially if they are not at high surgical risk.
ORGANIC TRICUSPID VALVE DISEASEOrganic TV disease is uncommon. In our setting, only 16% of patients requiring tricuspid surgery had organic valve disease, including those who had already undergone TV repair. In contrast, Rodríguez-Capitán et al.,1 found that almost 40% of patients in their series had organic valve disease, mostly of rheumatic origin. Rheumatic valve disease used to be the main cause of organic TV dysfunction but is currently unusual in our setting. In these patients, TR is often associated with stenosis, which is caused by retracted leaflets and/or decreased systolic excursion from subvalvular apparatus shortening. By contrast, degenerative TV disease is now increasingly common in patients who require surgery for mitral valve disease. Myxomatous TV degeneration is characterized by significant annular dilatation and redundancy of leaflets and subvalvular apparatus. Other causes of organic tricuspid dysfunction are extremely rare.
Indications, Techniques, and Outcomes of CorrectionSurgery in organic TR is usually indicated by associated left-sided valve disease. Correction of severe and/or symptomatic primary TV disease is only recommended when it is associated with left-sided value disease that requires surgery, or if it causes ventricular dysfunction.5,6
If the TV anatomy is sufficiently preserved, repair is preferable to replacement. Structural changes often make repairs complex to perform, but experienced groups manage to repair 60% to 70% of valves.12,13 Rheumatic TR repair sometimes requires opening of commissures and augmentation of leaflets. Edge-to-edge suturing can be useful in degenerative disease, because this type of suturing avoids the need to perform complex corrections to the subvalvular apparatus. The different underlying clinical and anatomical features and primary disease progression mean that the repair outcome is worse in organic than in functional TR. Long-time survival is shorter and the probability of valve dysfunction is higher, especially if no ring is used.14 Rodríguez-Capitán et al.1 also found a worse outcome in organic valve disease.
In more advanced cases, the only option is valve replacement, especially in reoperations on highly deformed valves. TV replacement surgery entails a higher risk and a worse outcome than left-sided valve replacements. Nevertheless, functional class improves in most patients, and survival is over 65% at 10 years.15 As Rodríguez-Capitán el al.1 show, prostheses guarantee valve competence, albeit with a substantial incidence of complications. There is no consensus on the ideal prosthesis for TV replacement. Both biological and mechanical prostheses carry a relatively high risk of thrombosis and need for reoperation. In the series of Rodríguez-Capitán et al., almost 25% of the patients who received a mechanical prosthesis developed prosthetic thrombosis. In contrast, other authors have found a worse hemodynamic outcome and a higher incidence of reoperation with bioprostheses.16 The type of prosthesis should be chosen according to the individual patient's characteristics (age, presence and type of other prostheses, etc.). The long-term outcome depends more on factors related to the valve disease itself than on the type of prosthesis used. In any event, a prosthesis is preferable to a poor repair, especially in the case of a second or third intervention indicated for residual or recurrent TR. When comparing the outcomes of TV replacement vs repair, the former entails lower mortality and a lower incidence of adverse events but has a higher incidence of residual TR and reoperation due to the persisting condition.17
The series of Rodríguez-Capitán et al.1 accurately reflects the situation to date in TR surgery in many Spanish hospitals; indications are restrictive and are limited to patients with more advanced or organic valve disease. This means that there have been relatively few cases during this long period of study; in fact, of a mean 133 patients undergoing valve surgery each year, TV interventions were only performed in 6.4%. Furthermore, because of the high proportion of patients with organic valve disease and/or previous surgery, surgery entails a high risk. Under these circumstances, the outcome is inevitably suboptimal, with a high mortality rate and poor functional outcome. Moreover, as the authors acknowledge, the retrospective design and the absence of clearly established criteria for deciding which repair technique or type of prosthesis to use make it hard to draw definitive conclusions.
CONFLICTS OF INTERESTNone declared.