The aim of this analysis was to evaluate the burden and cost of complications due to poor anticoagulation control in patients with nonvalvular atrial fibrillation (NVAF) treated with vitamin K antagonists (VKA) in Spain.
MethodsAn analytical model was used to estimate annual differences in ischemic stroke, major bleeding, deaths, costs, and potential years of life lost between patients with poor anticoagulation control (time in therapeutic range <65%) and adequate control (time in therapeutic range ≥ 65%) with a 1-year time horizon. Information on the target population (patients ≥ 65 years), event rates, and costs were obtained from national sources. Direct costs in euros (2018) were included from the perspective of the national health system (NHS) and direct and indirect costs from the societal perspective. A sensitivity analysis was performed with post-hoc data from the SPORTIF III/V trials.
ResultsWe analyzed a hypothetical cohort of 594 855 patients, 48.3% with poor anticoagulation control, with an increase of 2321 ischemic strokes, 2236 major bleeding events and 14 463 deaths, and an annual incremental cost between €29 578 306 from the NHS perspective and €75 737 451 from the societal perspective. The annual impact of mortality was 170 502 potential years of life lost. The results of the sensitivity analysis showed that the annual cost would reach €97 787 873 from the societal perspective.
ConclusionsPoor anticoagulation control with AVK has a strong impact on loss of health and on increased spending for the NHS.
Keywords
Oral anticoagulants are the treatment of choice for the prevention of strokes and mortality in patients with nonvalvular atrial fibrillation (NVAF) who are at significant thromboembolic risk according to the CHA2DS2-VASc scale.1 Although vitamin K antagonists (VKA) lower the risk of stroke by up to 64% compared with placebo,2 they require careful regular monitoring with frequent dosage adjustments due to a narrow therapeutic margin. There is solid evidence that patients with poor anticoagulation control throughout treatment (time in therapeutic range [TTR] <65% according to the Rosendaal method) are at higher risk of stroke, bleeding complications, and death3–6 than patients with adequate control.
In Spain, the prevalence of NVAF is around 4.4% in persons aged ≥ 40 years and rises gradually with age, reaching 17.7% in patients older than 80 years.7 According to the 2014 European PREFER registry on thromboembolic events, around 80% of patients with NVAF in Spain are receiving VKA therapy,8 although in recent years this percentage has dropped due to the use of new direct-acting anticoagulants.
Several publications have reviewed anticoagulant control in patients with NVAF and have identified a high number of patients with poor control, with a prevalence of 39.4% to 57.2% (TTR <65% according to the Rosendaal method),9,10 leading to a higher risk of stroke, bleeding complications, and all-cause mortality.11 In terms of economics, the CONOCES12 and CODICE13 studies provided data on the high cost of NVAF complications (stroke, hemorrhagic stroke, acute cardioembolic cerebral infarction, and major bleeding). Although several Spanish studies report data on the real-world situation of NVAF patients treated with VKA and the cost of their complications, no information is available on the potential socioeconomic impact of poor anticoagulant control in patients treated with VKA in the Spanish National Health Service (NHS).
The aim of this study was to evaluate the burden and cost of clinical events for the Spanish NHS that could arise due to poor anticoagulation control (determined by TTR according to the Rosendaal method) in NVAF patients treated with VKA.
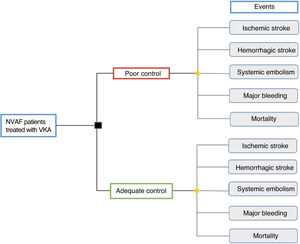
METHODSType of analysisA cost-outcome analysis was performed using an analytical model in Microsoft Excel 2013 to calculate the health outcomes (clinical outcomes) and the costs incurred for NVAF patients treated with VKA, according to the quality of anticoagulant control (figure 1). The results are expressed as the absolute difference in events and costs between patients with poor anticoagulant control (TTR <65%) and those with adequate control (TTR ≥ 65%). The analysis was performed from the NHS and societal perspectives, with a 1-year time horizon.
Target populationThe target population for the study consisted of patients aged ≥ 65 years with NVAF and treated with VKA in Spain. Information from the Spanish National Statistics Institute (INE)14 was consulted to determine the population aged ≥ 65 years, and the study applied the AF prevalence by age bracket reported by the OFRECE study7 and the proportion of NVAF patients with CHADS2 ≥ 2 treated with VKA reported by the VAL-FAAP study.15
Because the degree of anticoagulant control is a determining factor for prescribing direct-acting oral anticoagulants in the NHS, a structured literature review was undertaken to identify epidemiologic studies on the quality of anticoagulant therapy and event rates according to anticoagulant control in Spain. The studies were selected according to TTR by the Rosendaal method, and patients with poor control were defined as TTR <65% and those with adequate control as TTR ≥65%.16
Cohort characteristics (age, sex, and cardioembolic and bleeding risk according to the CHADS2, CHA2DS2-VASc, and HAS-BLED scales) and the percentage of patients with poor and adequate control were obtained by simple averaging of the values from the references.9,10,17–24 The extreme (lowest and highest) prevalences of anticoagulation control were used to define the range for clinical event outcomes and costs9,10 (table 1) to evaluate outcome variability. The study data considered and the cohort characteristics (age, sex, cardioembolic risk, and bleeding risk) are listed in .
Determination of the target population and event rate
| Determination of target population | |||||
|---|---|---|---|---|---|
| AF prevalence, segregated by age bracketa | |||||
| Age, y | Value | Reference | |||
| 65-69 | 4.60% | OFRECE study7 | |||
| 70-74 | 9.30% | OFRECE study7 | |||
| 75-79 | 9.30% | OFRECE study7 | |||
| 80-84 | 17.70% | OFRECE study7 | |||
| > 85 | 17.70% | OFRECE study7 | |||
| Proportion of patients with NVAF and CHADS2≥ 2 receiving VKA therapyb | |||||
| 68% | ValFAAP study15 | ||||
| TTR-based anticoagulation control in Spainc | |||||
| Poor control | Adequate control | Reference | |||
| 48.26% | 51.74% | Mean valued | |||
| Lowest prevalence value, poor control | 39.40% | 60.60% | PAULA study9 | ||
| Higher prevalence value, poor control | 57.20% | 42.80% | ESPARTA study10 | ||
| Event rates | |||||
| Poor control | Adequate control | Reference | |||
| BCc | SAe | BCc | SAe | ||
| Ischemic stroke | 2.02% | 1.84% | 1.13% | 1.02% | BC11; SA3 |
| Hemorrhagic stroke | ND | 0.20% | ND | 0.06% | BC11; SA3 |
| Systemic embolism | ND | 0.07% | ND | 0.00% | BC11; SA3 |
| Major bleeding | 3.03% | 3.85% | 2.10% | 1.58% | BC11; SA3 |
| All-cause mortality | 11.62% | 4.20% | 6.14% | 1.69% | BC11; SA3 |
AF, atrial fibrillation; BC, base case; INR, international normalized ratio; ND, no data; NVAF, nonvalvular atrial fibrillation; SA, sensitivity analysis; TTR, time in therapeutic range; VKA, vitamin K antagonists.
Poor control with INR <60% and adequate control with INR> 75%.
For the analysis, clinical events were stroke, major bleeding, and all-cause mortality. Clinical event rates derived from poor anticoagulant control were taken from a single-center study published in Spain,11 using a TTR value of 65% according to the Rosendaal method as a threshold between good and poor control (table 1).
A sensitivity analysis was also performed using event rates (ischemic stroke, hemorrhagic stroke, systemic embolism, major bleeding, and all-cause mortality) from a post hoc analysis of the SPORTIF III/V multicenter clinical trials, defining poor control as TTR <60% and good control as TTR >75%.3
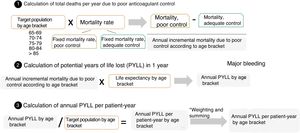
Impact of mortality: potential years of life lostAll-cause deaths in the comparison groups were measured in potential years of life lost (PYLL), by applying the mortality rate to the population aged ≥ 65 years with NVAF treated with VKA with poor anticoagulant control and adequate control by age bracket. Because there was no information according to age bracket, 2 fixed mortality rates were obtained: 1 for poor anticoagulant control and 1 for adequate control in each age bracket, based on the target population and according to the degree of anticoagulant control. Additionally, PYLL are expressed as the difference in mortality between patients with poor control and adequate control, multiplied by the mean life expectancy for each age bracket, taken from National Statistics Institute sources. Last, PYLL and PYLL/patient-year data were obtained by converting the PYLL results for each age bracket divided by the age-adjusted population, ie, each age bracket was proportionally represented according to the weight of the various age brackets compared with the entire target population (figure 2).
CostsAll clinical events, except all-cause mortality, led to costs and were updated to year 2018 euros based on the consumer price index (CPI) increase since the cost year reported in the original source.25 Direct health, direct nonhealth, and indirect costs of ischemic and hemorrhagic stroke were evaluated from data published by CONOCES,12 an epidemiologic study of first-year costs in patients admitted to stroke units in Spain.
The cost of major bleeding was determined from diagnosis-related groups 174 (gastrointestinal bleeding with complications), 175 (gastrointestinal bleeding without complications), and 810 (intracranial bleeding).26 The proportions of major bleeding (73.7% gastrointestinal bleeding and 26.3% intracranial bleeding) were taken from a study that analyzed bleeds in patients with NVAF and CHADS2 ≥ 2 treated with VKA listed in the National Primary Care Database (SIDIAP)27 (table 2).
Unit costs used in the model
| Costs (2018 euros) | Reference | |
|---|---|---|
| Ischemic stroke | ||
| Direct healtha | 9037.22 | CONOCES study12 |
| Direct nonhealthb | 19 259.83 | CONOCES study12 |
| Indirectc | 624.01 | CONOCES study12 |
| Hemorrhagic stroke | ||
| Direct healtha | 7467.84 | CONOCES study12 |
| Direct nonhealthb | 21 389.60 | CONOCES study12 |
| Indirectc | 397.87 | CONOCES study12 |
| Systemic embolism | ||
| Direct health | 3846.18 | Escolar-Albaladejo et al.28 |
| Major bleeding | ||
| Direct health | 2982.61 | DRG-MSCyBS26,27 |
DRG, diagnosis-related groups; MSCyBS, Ministry of Health, Consumer Affairs and Social Welfare.
Hospitalization cost for the first stroke episode, rehospitalizations during the entire follow-up period related to the stroke or new vascular events (1 year), medical visits, diagnostic procedures and tests, medical treatments, and rehabilitation.
The model structure and the parameters included were validated by an expert panel composed of 4 medical specialists related to management of the disease (1 cardiologist, 1 hematologist, and 2 primary care physicians).
RESULTSThe hypothetical cohort for this study included 594 855 patients with a mean age of 75.4±4.3 years (48.9% women) at high cardioembolic and bleeding risk: 74.4% with CHADS2 ≥ 2 (91.8% if CHA2DS2-VASc scale ≥ 2 is used) and 58.4% with HAS-BLED ≥ 2. The mean prevalence of poor anticoagulant control was 48.3% (n=287 089), between 39.4% (n=234 373) and 57.2% (n=340 257).
Compared with adequate control, poor anticoagulant control was associated with an increase of 4557 events per year (2321 ischemic strokes and 2236 major bleeds) and 14 463 all-cause deaths (table 3).
Number of events per year (base case and sensitivity analysis)
| Base case | Poor control (range)a | Adequate control (range)b | Difference between poor control and adequate control (range)c |
|---|---|---|---|
| Ischemic stroke | 5799 (4734-6873) | 3478 (4073-2877) | 2321 (661-3996) |
| Hemorrhagic stroke | ND | ND | ND |
| Systemic embolism | ND | ND | ND |
| Major bleeding | 8699 (7101-10 310) | 6463 (7570-5347) | 2236 (–469 to 4963) |
| Deathsd | 33 360 (27 234-39 538) | 18 897 (22 134-15 632) | 14 463 (5101-23 906) |
| Sensitivity analysis | Poor control (range)e | Adequate control (range)f | |
| Ischemic stroke | 5282 (4312-6261) | 3139 (3677-2597) | 2143 (636-3664) |
| Hemorrhagic stroke | 574 (469-681) | 185 (216-153) | 390 (252-5287) |
| Systemic embolism | 201 (164-238) | 0 | 201 (164-238) |
| Major bleeding | 11 053 (9023-13 100) | 4863 (5696-4023) | 6190 (3328-9077) |
| Deathsd | 12 058 (9844-14 291) | 5201 (6092-4303) | 6856 (3752-9988) |
ND, no data; TTR, time in therapeutic range.
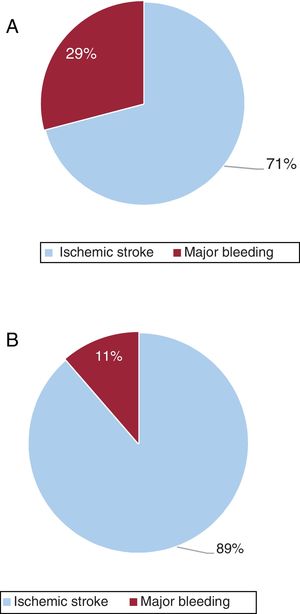
The annual cost of events due to poor anticoagulation control, considering only direct health costs, was €29 578 306 (€116 /patient-year), mainly due to the cost associated with ischemic stroke (€20 979 365/year), which accounted for 70.9% of event costs.
When considering the societal perspective (including direct nonhealth costs and indirect costs), the impact of poor anticoagulant control was €75 737 451 (€293/patient-year), with ischemic stroke accounting for an even larger portion of the cost (€67 138 510/year; 88.6%) (table 4 and figure 3). The impact on mortality for the entire population analyzed was 170 502.05 PYLL (0.287 PYLL/patient-year).
Costs of poor and adequate control of anticoagulant therapy (base case)
| Poor control (range)a | Adequate control (range)b | Difference between poor control and adequate control (range)c | |
|---|---|---|---|
| NHS perspective | |||
| Ischemic stroke | 52 408 627 (42 785 212-62 114 571) | 31 429 261 (36 812 657-25 999 698) | 20 979 365 (5 972 555-36 114 873) |
| Major bleeding | 33 457 172 (27 313 675-39 653 355) | 24 858 231 (29 116 100-20 563 846) | 8 598 941 (1 802 425-19 089 509) |
| Total | 85 865 798 (70 098 887-101 767 927) | 56 287 492.18 (65 928 757-46 563 544) | 29 578 306 (4 170 130-55 204 382) |
| Societal perspective | |||
| Ischemic stroke | 167 718 948 (136 921 938-198 780 072) | 100 580 439 (117 808 469-83 204 661) | 67 138 510 (19 113 468-115 575 411) |
| Major bleeding | 33 457 172 (27 313 675-39 653 355) | 24 858 231 (29 116 100-20 563 846) | 8 598 941 (–1 802 425 to 19 089 509) |
| Total | 201 176 120 (164 235 613-238 433 428) | 125 438 669 (146 924 569-103 768 508) | 75 737 451 (17 311 043-134 664 920) |
NHS, National Health Service; TTR, time in therapeutic range.
Costs expressed in euros (2018).
The sensitivity analysis performed with event rates from a national study showed consistent results with the base-case analysis, and the total annual number of clinical events was an additional 8924 (2143 ischemic strokes, 390 hemorrhagic strokes, 201 systemic embolisms, 6190 major bleeds) and 6856 all-cause deaths among patients with poor control, compared with patients with adequate control (table 3). The analysis showed an increase in the number of major bleeds, although a considerable decrease in all-cause mortality was associated with poor anticoagulant control compared with the base case (14 463 all-cause deaths) (table 3).
The annual cost of the impact of events was €46 685 757 (€174/patient-year) according to the NHS perspective and €97 787 873 per year (€368/patient-year) according to the societal perspective (). The cost of major bleeds represented a larger portion of the cost of poor control (51%) from the NHS perspective, whereas ischemic stroke was the main contributor (63%) from the societal perspective (). The impact of mortality was 80 830.65 PYLL (0.136 PYLL/patient-year).
DISCUSSIONThe results of this study, the first in Spain to analyze the costs associated with oral anticoagulation quality, reveal the clinical and economic impact of poor control among patients receiving VKA therapy, a common clinical situation in Spain9,10,17–24 and a risk factor for stroke in patients with NVAF.8 Another relevant finding of the analysis is the estimated mortality among these patients, expressed as PYLL.
The analysis started with the prevalence figures according to age bracket from the OFRECE,7 CARDIOTENS,29 PREV-ICTUS,30 and AFABE31 studies, but the prevalence data from Gómez-Doblas et al.7 were finally used because the study population was the most relevant. The percentage of VKA use was taken from the Val-FAAP study,15 which described a population receiving oral anticoagulants in a similar sample with similar characteristics to the cohort analyzed (population with CHADS2 ≥ 2, 74.4%). Although this study did not specify the type of anticoagulant therapy, it is assumed to be VKA in all patients because the study was conducted between 2009 and 2010, when direct-acting oral anticoagulants were rarely used. However, although the European PREFER registry has reported on VKA usage in Spain (80%),8 the present study was finally based on the levels from Barrios et al.,15 because the patients in this study were at high cardioembolic risk (CHADS2 ≥ 2), similar to the cohort analyzed.
This analysis included studies evaluating the quality of anticoagulant therapy based on TTR according to the Rosendaal method using 65% as a cutoff point, as the method is considered most orthodox, is widely used in our field,21 and is recommended by health authorities.32 The PAULA9 and ESPARTA10 studies provided the extreme values for the analysis.
Despite the magnitude of the levels analyzed, the estimated impact of poor anticoagulant control could be lower than the real-world impact. According to the AFABE study31 results, 23.5% of patients with known AF and moderate-to-high cardioembolic risk (CHA2DS2-VASc ≥ 2) did not receive anticoagulant therapy and, therefore, a large patient population would not be included in the analysis. However, no cost was assigned to mortality, as no data were found on the amounts. In addition, the direct nonhealth and indirect costs of systemic embolism and major bleeding have not been identified and, therefore, these costs were not included in the analysis.
The limitations of this analysis include aspects related to calculating the target population (NVAF prevalence) and event rates. First, prevalences were applied to each age bracket (10-year intervals) from the OFRECE study7 to the population prevalences taken from the Spanish Statistics Institute (5-year intervals), which could underestimate the prevalences in the older groups of each bracket. Second, in the real-world study conducted in Spain by Rivera et al.,11 event rates were limited to ischemic stroke, major bleeding, and all-cause mortality, unlike the study used in the sensitivity analysis (post hoc analysis of the SPORTIF III and V clinical trials performed in 23 countries in Europe, Asia and Oceania, United States, and Canada),3 which also included ischemic stroke and systemic embolism rates. Third, questions could be raised regarding the suitability of the White et al.3 study for the sensitivity analysis, as it was an international multicenter study with a different definition of poor anticoagulant control from ours (poor control, TTR <60%; good control, TTR> 75%). However, the differences in rates used in the 2 studies were very similar between the groups (difference between adequate and poor control in ischemic stroke, 0.89% vs 0.82%; major bleeding, 0.93% vs 2.27%; mortality, 5.48% vs 2.51% in the base case and sensitivity analysis, respectively). Hence, it can be stated that the event and mortality rates used are valid in our model. Nevertheless, although the prevalence of NVAF has been applied by age bracket, the same exercise could not have been undertaken with the prevalence of poor control and the event rates because the information was not segregated by age. Furthermore, it would have been interesting to evaluate the clinical and economic impact of poor anticoagulant control according to possible differences between various regions of Spain, as mentioned in the PAULA study.33 Last, the analysis was limited to a 1-year time horizon, considering the 1-year unit costs of ischemic stroke and hemorrhage for these events (CONOCES study),12 which may not be representative of the entire natural course of the disease.
Despite the above-mentioned limitations, the study results underscore the high socioeconomic impact of poor control of VKA therapy in Spain, essentially based on acenocoumarol (although no differences were observed in the quality of anticoagulation control or events compared with warfarin).34 This poor control may have various causes: the presence of comorbidities (eg, kidney failure, type 2 diabetes mellitus, hypertension), factors such as labile international normalized ratio (INR), high bleeding risk, female sex, dietary habits, multidrug therapy, and prolonged use of antiplatelet agents.10,17,21,35 Obviously, nonadherence is a key factor (as with all long-term therapy), as observed in the REACT-AF study, which observed adherence> 80% in only 34.9% of patients.36 The last possible cause is therapeutic inertia, reported in studies such as ESPARTA, in which therapy was not switched to a direct-acting oral anticoagulant in 65.6% of NVAF patients at high risk of stroke (CHA2DS2-VASc ≥ 2) and treated with VKA who did not maintain INR within therapeutic range.10
Irrespective of these considerations, poor anticoagulant control in Spain, in addition to affecting NVAF patients by compromising their clinical prognosis and producing higher mortality and disability, makes the NHS less efficient. The results of this study reveal the clinical and economic impact of poor anticoagulant control in NVAF patients treated with VKA, highlighting the potential importance of a change in clinical practice to lower the disease burden.
CONCLUSIONSThis study shows that higher rates of thrombotic, bleeding events, and mortality among patients with poor anticoagulant control treated with VKA represent a cost of more than €75 million per year (from the societal perspective) and a high impact of mortality, expressed in PYLL (up to 170 502 per year). Because this is a major issue for patients and the NHS, measures should be implemented to reduce the number of patients receiving VKA therapy who have poor anticoagulant control, thus minimizing the socioeconomic impact in our setting.
FUNDINGBMS and Pfizer provided unconditional funds for this project.
CONFLICTS OF INTERESTV. Barrios, S. Cinza-Sanjurjo, O. Gavín, and I. Egocheaga declare no conflicts of interest. R. Burgos-Pol and M.A. Casado are employees of PORIB. C. Polanco and J. Suárez are employees of BMS. J. Soto is an employee of Pfizer.
- –
VKA therapy is indicated in patients with NVAF and high cardioembolic risk.
- –
VKA therapy requires monitoring and dose titration. The time in therapeutic range, as measured by the Rosendaal method, evaluates the quality of monitoring of VKA anticoagulation and correlates with the appearance of thromboembolic or bleeding events.
- –
Numerous studies have shown that a high percentage of patients are not properly anticoagulated, a situation associated with a higher incidence of thromboembolic events, bleeds, and mortality.
- –
This study is a cost-impact model based on real-world studies, providing clinical and economic information on the high impact of poor control of VKA anticoagulation in Spain.
- –
Clinical events could be as high as 4557, with a cost of €30 to 75 million per year, according to the perspective used.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.06.033