Keywords
INTRODUCTION
Familial hypercholesterolemia (FH) is a common genetic disorder transmitted by an autosomal dominant gene, and which affects the receptor for low density lipoprotein LDL.1 It is estimated to affect one in every 400-500 people in the general population and is the most common monogenic disorder associated with the development of premature cardiovascular disease. It has been shown that 50% of males and 20% of women with heterozygous FH who do not receive suitable treatment will suffer an acute coronary episode in their fifties.2-4 An increased risk of fatal coronary events has also been observed in people with FH under 40 years of age.5 However, since statins were introduced, mortality has decreased in FH particularly in those under 60 years6 Early diagnosis and appropriate treatment are therefore essential in preventing cardiovascular disease associated with FH.7 Several studies have shown that the most cost-effective preventive strategy is that of screening the close relatives of individuals diagnosed with FH.8,9 The results are robust to variations in the discount rates used, to medication costs (statins), cardiovascular events, the test used, and other parameters included in the analysis.8-10 After diagnosis, most people begin drug treatment to control low-density lipoprotein cholesterol (LDL-C) and reduce the risk of a future cardiovascular episode.11 A new tool for the diagnosis of genetic FH based on DNA array has been available in Spain since 2004.12 This method provides a highly sensitive and specific analysis of the low density lipoprotein gene receptor (LDLR) relatively quickly.12
The aim of the present study was to assess the cost-effectiveness of a genetic screening program for first-degree relatives of patients with familial hypercholesterolemia (FH), followed by treatment when necessary, compared with the alternative of no screening.
METHODS
We performed a cost-effectiveness analysis in which the target strategy was the genetic screening, and subsequent treatment with statins, of the immediate family members (parents, siblings, children) of patients with a prior genetic diagnosis of FH. The alternative strategy was no screening. Both strategies included follow-up and treatment of individuals who had had a cardiovascular event. The analysis was performed from the perspective of the National Health System (payer perspective). The main effectiveness outcome was life year gained (LYG) for each new case detected and treated with statins as a result of diagnosis.
Genetic Diagnosis Program in Index Cases and Screening of Family Members
Data from a pilot study conducted by the Foundation for Family Hypercholesterolemia was used in the analysis of the genetic screening program in Spain.12 In this study, index cases with suspected FH were identified using a uniform protocol for clinical diagnosis.13 Genetic analysis was performed using the Lipochip® platform, which includes three diagnostic procedures performed sequentially: a) DNA array, which includes the more frequent LDLR mutations in Spain and which is regularly updated; b) if the DNA array analysis is negative, multiplex quantitative PCR is used to identify significant rearrangements, and c) if the 2 previous analysis are negative, the sample is analyzed through complete sequencing of the LDLR gene. Study results showed that the clinical diagnosis was correct in approximately 59% of cases when FH was suspected, but that the detection rate was much higher (72%) when the clinical diagnosis was certain. The DNA array had a specificity and sensitivity of 99.7% and 99.9%, respectively.12
Only steps 1 or 2 of the diagnostic procedure were carried out in the study of family members, depending on the mutation present in the index case (specific mutation or large rearrangements). This information was used when calculating the cost of screening for family members. To do this, we assumed that index cases were diagnosed genetically and that genetic screening was then performed in family members. This assumption is reflected in the sensitivity analysis to take into account the case of first-degree relatives of genetically diagnosed patients.
Analytical Framework
The distribution by age and sex of patients and their families was obtained from the cohort study in families with FH conducted by the Familial Hypercholesterolemia Foundation. Specifically, we used the age and sex distribution of 503 patients under 60 years of age from that cohort.3,13 Age- and sex-adjusted life expectancy data from the National Statistics Institute (INE) were used to apply the relative risks in the literature6,10 for patients diagnosed with FH and treated with statins and those not diagnosed with FH.
Effectiveness: Years of Life Gained
Mortality rates were calculated based on specific national mortality rates by age and sex for the general population. To determine the survival probability of patients with FH (with and without statin treatment), specific relative risks were calculated by age and sex based on Wonderling et al methodology.10
Data from the screening program was supplemented with data from the United Kingdom's Simon Broome Register.6 This is a cohort of 1185 patients with heterozygous FH followed prospectively since 1980 who have been treated primarily with statins from 1992 until completion of analysis. To our knowledge, this is the only cohort of patients with FH which has been studied over a sufficiently long period of time to be able to compare the situation of patients before and after statin treatment. For FH patients under 20 years and those over 60, treatment with statins had no significant effect on the estimated mortality risk, so health benefits are not applied to patients over 60 years of age in our models.
Health Care Costs
Statins (HMG-CoA reductase inhibitors) are the drugs of choice in the treatment of hypercholesterolemia. A daily dose of 40 mg/day was considered appropriate for two types of statin (simvastatin and atorvastatin) to achieve lipid control in patients with FH. The average annual cost of treatment was calculated using market prices (RRP+VAT).
The base case used the Ministry of Health and Consumer Affairs reference price for simvastatin 40 mg. The lowest prices for 40 mg of simvastatin and 40 mg of atorvastatin were used in the sensitivity analysis. The average annual cost of treatment with statins in the base case was 282.5 euros. It was assumed that treatment would also include two annual visits to a specialist. The unit cost assigned to these visits was 55 euros, which is the unit cost of a visit to a cardiologist.14 The annual cost of treatment with statins plus 2 visits to a specialist was therefore 392.5 euros. Costs were expressed in 2005 euros. Both patients who receive treatment with statins and those who do not are at risk of suffering an acute cardiovascular event, though the risk is greater in the latter group. In addition, cases of myocardial infarction and the unit cost of each case were estimated based on diagnosis related group (DRG) 121.15
The total cost of treatment and events avoided was calculated based on INE survival tables and adapted by applying the relative risk of mortality in patients treated with statins from the Simon Broome cohort, using the same methodology.10 The incidence of fatal myocardial infarctions was obtained from national data for Spain; the relative risk from the Simon Broome cohort was used to determine all causes of death. It was assumed that there would be 1.4 non-fatal acute myocardial infarctions (AMI) for each fatal AMI in men and 1.2 non-fatal AMIs for each fatal AMI in women.10
Once the patient was diagnosed with FH, it was assumed that there would be a reduction in the risk of a cardiovascular event and, therefore, a gain in life years, provided that the patient was treated appropriately and the condition was well-controlled. Diagnosis and treatment does not mean that patients' age- and sex-adjusted mortality risk becomes the same as that of the general population, but that the mortality risk in the population identified and treated is lower than that in an untreated population with FH.6,10
Cost of Screening Plus Treatment
To calculate the cost of screening family members, the results of the pilot study mentioned above were taken into account.12 Thus, to detect one positive case of FH in a first-degree relative, a total of 3.4 screenings would be required. As the cost of a screening is 425 euros including taxes (data source: Progenika SA), and as the strategy is to screen all of the patient's relatives and it is assumed that 1 in 2 (50%) also have familial hypercholesterolemia, the cost per positive case is 1447 euros.
Cost per Life Year Gained
The incremental cost per LYG was calculated as the cost of screening plus patient treatment less savings resulting from a reduction in the incidence of coronary events, all divided by LYGs. In the base case, a discount rate of 3% per annum was applied to both costs and health effects.
Sensitivity Analysis
To verify the robustness of the base case, several types of univariate sensitivity analysis were performed, together with a probabilistic analysis. The model performed 5000 Monte Carlo type simulations. The result of each simulation was an incremental cost-effectiveness ratio (ICER) derived from incremental costs and outcomes.
RESULTS
Life Years Gained
Using data on relative risks from the Simon Broome cohort6 applied to mortality rates in Spain, it was estimated that the life expectancy for a 20 year old male with heterozygotic FH treated with statins from the age of 20 would be 70.6 years. Life expectancy in this case without statin treatment would be 65.6 years. The figures for a 20 year old female were 77.2 years without treatment and 82.3 years with treatment. The LYGs with treatment varied according to age at diagnosis (Table 1). New cases diagnosed by the screening program were expected to gain a mean of 3.3 years each (1.3 years when a discount rate of 3% was applied).
Incremental Costs
The incremental cost of the screening program includes the cost of screening plus the cost of drugs and 2 annual visits to a specialist minus the cost of savings associated with a reduction in coronary events. The cost of screening for each new case was 1447 euros, while the mean expected cost for treatment and clinic visits was 4529 euros. It was estimated that 26 AMI would be avoided per 100 people treated with statins between the ages of 18 and 60.10 Therefore, savings per AMI avoided per diagnosed individual (1384 euros) largely offset the cost of the screening program, but not the cost of treatment.
Cost-Effectiveness
Dividing the total incremental cost per additional LYG gave an incremental ratio of 3423 euros / LYG (costs and years of life discounted at 3%). Thus, in comparison to an alternative strategy of no screening, a genetic screening program for first-degree relatives of patients diagnosed with FH would require an investment of 3423 euros for each additional LYG (Table 2).
Sensitivity Analysis
We performed a univariate sensitivity analysis in the deterministic model to evaluate its impact on cost and effectiveness of screening. Choice of discount rate significantly affected incremental cost-effectiveness, from a minimum (best outcome) of 1073 euros / LYG to a maximum (worst outcome) of 5206 euros / LYG (Table 3). However, in all of these situations, the incremental cost-effectiveness of the genetic screening program in first-degree relatives was very favorable when compared to the alternative of not screening, according to the criteria commonly used in Spain.16 Therefore, the results can be considered to be robust to changes in the discount rate.
Several analyses were performed in which the costs used in the base case analysis were varied. When only variables related to costs were modified, LYGs remained constant. Table 4 shows that the results would only be significantly different if all patients were treated with atorvastatin. In this case, the incremental ratio would be 9708 euros / LYG. If patients were treated with the lowest-priced simvastatin, the incremental ratio would be 2569 euros / LYG, whereas if we do not consider any savings from the prevention of heart attacks the incremental ratio would be 4454 euros / LYG.
Moreover, if all patients were correctly diagnosed with FH because genetic diagnosis was used in all cases, the cost of detecting one case of FH among relatives would be equal to the cost of the test (425 euros) multiplied by the probability that a family member actually had FH. Assuming that the probability is 50%, the cost of detecting one case of FH would drop to 850 euros and the incremental cost of a LYG would be close to 3000 euros / LYG.
Probabilistic Analysis
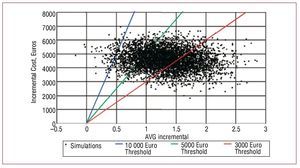
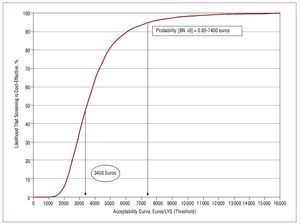
The distributions and parameters used in the probabilistic analysis are shown in Table 5. The results are supplemented by the mapping of the cost-effectiveness plane and the acceptability curve. The cost-effectiveness plane (Figure 1) shows the incremental cost and outcome of each simulation.17 The acceptability curve (Figure 2) describes the likelihood that intervention x is optimal, given the data generated in the stochastic analysis. The acceptability curve can therefore be interpreted as the likelihood that the intervention x is cost-effective.18
Figure 1. The cost-effectiveness plane shows that most of the points appear in the quadrant representing higher cost and greater effectiveness (4997 of 5000 simulations performed). Most items are below the boundary of the 10 000 euros / incremental LYG. The number of points below the 3000 euros / incremental LYG threshold is also high. LYG indicates life years gained.
Figure 2. The acceptability curve shows that if societal or payer willingness to pay of was 7400 euros per incremental LYG then the likelihood that the screening strategy was optimal, compared to the alternative of not screening, would be 95%. If willingness to pay was greater than or equal to 15 000 euros / incremental LYG then the likelihood would be close to 100%.
Finally, we calculated the expected value of perfect information (EVPI)19 from the net benefit. The EVPI quantifies how the cost of uncertainty in the model affects incremental cost-effectiveness. For each threshold (maximum willingness to pay), we obtained the maximum amount we would be willing to pay to get better information (perfect information) to inform our decision-making given the target population. The EVPI curve reached its maximum value of 650 euros per person when maximum willingness to pay for one additional LYG was approximately 3500 euros (cost-effectiveness point). Beyond that point, the EVPI became considerably lower; for example, at a threshold of 10 000 euros the EVPI was 30 euros.
DISCUSSION
This study has shown that the consistent implementation of genetic screening in relatives of patients previously diagnosed with FH is cost-effective. The results are especially favorable when compared with other health care interventions, and the findings of our study are similar to those of economic evaluations conducted in the UK and The Netherlands.8-10 It should be noted that, although the cost-effectiveness ratio is higher because of the inclusion of treatment with atorvastatin, the result is based on a conservative estimate. That is, we have assumed the same effect on LYG even though the doses of simvastatin and atorvastatin used are not equipotent. We retained this case to show that even when a more expensive statin is included, the cost per LYG remains attractive. However, a recent study showed that treatment of FH using atorvastatin monotherapy up to 80 mg/day, and even in combination with ezetimibe, was also cost-effective.20 Optimal pharmaceutical treatment for the disorder is therefore an aspect to consider in future analysis.
People who suffer from FH have age- and sex-adjusted mortality rates which are between 4 and 5 times higher than those of the general population,6,21 and the identification of the FH is a prerequisite for correct treatment. Clinical diagnosis of FH is essentially based on the concentrations of LDL-C and family history of hypercholesterolemia and premature cardiovascular disease. Levels of LDL-C cannot be considered the standard for a diagnosis of FH due to problems of sensitivity and specificity, with values which sometimes overlap those of the general population. It has been shown that using LDL-C leads to a diagnostic error rate of 17% in carriers of a single functional mutation and of 12.5% in those who are not affected by FH.22 Moreover, concentrations of LDL-C appear to be a poor predictor of FH in family members, as 23.5% of relatives with a mutation have levels of LDL-C which are below the 90th percentile and 15% of unaffected individuals have levels which are above this percentile.23
Our study likely underestimates the health benefits that would result from the implementation of an FH detection and treatment program, as the results of the Simon Broome cohort came from patients treated with statins from 1992 until completion of their study, which was published in 1999. The patients were generally treated with lower doses than those which would be indicated today.24-26 Therefore, the gains in terms of cardiovascular events and premature deaths avoided, and thus in LYG, are likely to be lower in our study than they would be reality.
Moreover, the results would be more favorable in terms of LYG if FH was diagnosed at a younger age. In Spain, over 50% of cases in both men and women were diagnosed over the age of 50 (from the Spanish FH cohort, data not shown). This has important economic and health implications, because the health benefit (LYG) is greater the earlier the diagnosis of FH.10 These results should therefore be interpreted as a conservative estimate.
Study Limitations
Certain difficulties were encountered when trying to estimate health outcomes for this study. There are no randomized clinical trials (RCTs) available which compare the results of FH patients treated with statins with those of untreated patients with the same disease. Such trials will likely never be performed as they would be ethically unacceptable in this population, given the well-known benefits of statin therapy in FH.6,24-26
On the other hand, the correct diagnosis of patients with FH could lead to the performance of clinical trials and observational cohort studies which compare effectiveness in the use of different statins, different therapeutic doses, and other medication which could be used to complement statins. The effect of other cardiovascular risk factors such as diet, exercise, smoking, etc, should also be studied.
Given the perspective of the analysis, it was appropriate to focus on direct health costs, although other costs such as those associated with general and local health care organization or training of personnel could have been included. However, this would not change the conclusions of the present analysis. Moreover, the implementation of a selective genetic screening program in relatives of patients with FH, and screening of a wider group of people, could lead to benefits in the form of social costs avoided, such as those associated with lost productivity. In a cost-effectiveness analysis of simvastatin for the treatment of patients with low cholesterol and heart disease, Johannesson et al27 estimated an incremental cost per LYG of between 3800 dollars and 27 000 dollars, depending on the levels of cholesterol, and the patient's age and sex. When avoidable productivity losses were included, the results were much more favorable, ranging from a net cost saving plus the benefits of therapy to a cost-effectiveness ratio of 13 000 dollars per LYG. Indeed, ischemic disease leads to huge productivity losses in Spain.28,29 Future analyses in this area could therefore usefully be performed from the social perspective by including all social costs. It would likewise be recommendable to use quality adjusted life years as the final endpoint. Unfortunately, the available data did not allow us to incorporate such an outcome.
CONCLUSIONS
Genetic screening for first-degree relatives of people diagnosed with FH, and subsequent treatment, is an efficient alternative when compared with the alternative of no screening. The use of sensitivity analysis indicates that the results obtained are robust to changes in the parameters used, whilst the use of probabilistic simulation analysis helps to clarify the study results. In this case, the incremental ratio of the technology in question is 3423 euros per LYG, which is very reasonable. Similarly, using information provided by the sensitivity analysis, we note that if the acceptability threshold in Spain lies at about 7500 euros, the strategy of genetic screening plus treatment first-degree relatives of people diagnosed with FH would have a 95% probability of being efficient.
In Spain, genetic detection of FH is being performed in some regions by medical specialists. To date, approximately 5000 genetic analysis have been conducted with a detection rate for index cases of around 65%. It has been shown that genetic screening identifies patients at a younger age and that it improves treatment and treatment compliance.22 The results obtained therefore provide support for the implementation of a plan for the detection of FH.
ACKNOWLEDGMENTS
Julio Lopez and Juan Oliva acknowledge the support of the CIBER in Epidemiology and Public Health (CIBERESP).
Pedro Mata and Rodrigo Alonso gratefully acknowledge the support of the Genetic Hyperlipidemia Network (G03/181).
This work was performed in part with financial assistance from Lacer SA, and data provided by the Family Foundation Hypercholesterolaemia.
ABBREVIATIONS
AMI: acute myocardial infarction
FH: familial hypercholesterolemia
LDL: low density lipoprotein
LDLR: low density lipoprotein receptor
LYG: life year gained.
Correspondence: Dr. J. Oliva.
Departamento de Análisis Económico y Finanzas. Facultad de Ciencias Jurídicas y Sociales de Toledo. Universidad de Castilla-La Mancha.
Cobertizo de San Pedro Mártir, s/n. 45071 Toledo. España.
E-mail: juan.olivamoreno@uclm.es
Received April 15, 2008.
Accepted for publication October 21, 2008.