Implantable cardioverter-defibrillators (ICD) are a cost-effective alternative for secondary prevention of sudden cardiac death, but their efficiency in primary prevention, especially among patients with nonischemic heart disease, is still uncertain.
MethodsWe performed a cost-effectiveness analysis of ICD plus conventional medical treatment (CMT) vs CMT for primary prevention of cardiac arrhythmias from the perspective of the national health service. We simulated the course of the disease by using Markov models in patients with ischemic and nonischemic heart disease. The parameters of the model were based on the results obtained from a meta-analysis of clinical trials published between 1996 and 2018 comparing ICD plus CMT vs CMT, the safety results of the DANISH trial, and analysis of real-world clinical practice in a tertiary hospital.
ResultsWe estimated that ICD reduced the likelihood of all-cause death in patients with ischemic heart disease (HR, 0.70; 95%CI, 0.58-0.85) and in those with nonischemic heart disease (HR, 0.79; 95%CI, 0.66–0.96). The incremental cost-effectiveness ratio (ICER) estimated with probabilistic analysis was €19 171/quality adjusted life year (QALY) in patients with ischemic heart disease and €31 084/QALY in those with nonischemic dilated myocardiopathy overall and €23 230/QALY in patients younger than 68 years.
ConclusionsThe efficiency of single-lead ICD systems has improved in the last decade, and these devices are cost-effective in patients with ischemic and nonischemic left ventricular dysfunction younger than 68 years, assuming willingness to pay as €25 000/QALY. For older nonischemic patients, the ICER was around €30 000/QALY.
Keywords
The implantable cardioverter-defibrillator (ICD) is an effective device used to treat life-threatening ventricular arrhythmia.1 More than a decade after the publication of the earliest pivotal trials,2,3 the efficacy of ICD for primary prevention of sudden cardiac death has been a subject of debate due to the publication of the last clinical trial performed in patients with nonischemic heart failure.4 This debate led to the publication of numerous systematic reviews and meta-analyses, concluding that ICD in primary prevention improves the survival of patients with ischemic5,6 and nonischemic6–17 heart failure and ejection fraction (EF) ≤ 35% compared with conventional medical treatment (CMT).
According to the official registry published in Revista Española de Cardiología,18 the prophylactic use of ICD accounts for 62% of all indications, with high variability between hospitals, and is the fastest-growing indication for patients with dilated cardiomyopathy: 63.5% of indications vs 49.9% in patients with ischemic heart disease.
Health technology assessment is a basic tool used in decision-making and resource allocation to ensure high quality services and system sustainability.19 A technology review report prepared in our setting in 201120 concluded that ICDs were a cost-effective alternative for primary prevention in patients with ischemic dilated cardiomyopathy who met the criteria of the MADIT trial.21 Conversely, the indication of ICD in primary prevention was not cost-effective, assuming willingness to pay as €30 000/quality-adjusted life year (QALY), in patients who met the MADIT II2 criteria and had nonischemic dilated cardiomyopathy.
There is evidence22,23 that the arrhythmia mortality rate and the rate of appropriate therapies have dropped in recent years and that they vary according to age and comorbidity. Consequently, many patients with ICD will never receive an appropriate discharge or will die due to a nonarrhythmic cause.24 A secondary finding25 of the DANISH study was that the analyses of predefined subgroups observed a positive effect of ICD in terms of reducing the risk of mortality among patients with nonischemic ventricular dysfunction who received an ICD at earlier ages. Therefore, it is essential to select patients with a higher expected benefit of the ICD and a lower probability of complications to allow an efficient use of ICD, particularly in indications for primary prevention.
The aim of this study was to evaluate the cost-utility of ICD in primary prevention for patients receiving CMT, updating the 2011 review with any new evidence, and to determine in which patient subgroups it would be cost-effective according to age and type of heart disease.
METHODSCost-utility economic model based on a Markov decision analysis.
Decision modelA Markov model was used to estimate the costs and survival of patients on CMT who receive an ICD for primary prevention and those who do not. A cost-utility analysis was performed from the perspective of the national health service, using patient lifetime as the time frame and applying a discount rate of 3% per year for both costs and benefits. The analysis considered CMT vs ICD plus CMT for primary prevention in patients with 3 different profiles: a) ischemic ventricular dysfunction; b) nonischemic ventricular dysfunction, and c) nonischemic ventricular dysfunction in patients age 68 years or less (predefined subgroup of the DANISH study seen to benefit from the ICD).
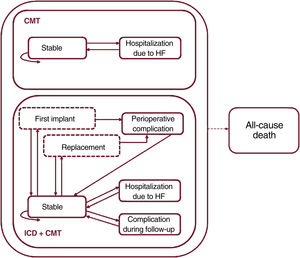
The model used in a previous evaluation26 was adapted with the TreeAge Pro software (version 2019 R2.0). A hypothetical cohort was simulated of 60-year-old patients with functional class II-III heart failure and EF<35%, with or without a history of ischemic heart disease, who received a single-chamber ICD (CMT+ICD group) for primary prevention or who did not receive it (CMT group). Figure 1 shows the various states through which patients could move in monthly cycles and the transitions between them. All patients started with a “stable” state and, from there, were at particular risk of all-cause death or hospitalization for heart failure in each month. Additionally, patients in the CMT+ICD group could experience a device-related complication at any time or a perioperative complication in the first month or when the battery was replaced (assumed to be 8 years after the first implant).
Markov model structure. Health states are shown as solid-line boxes, and transition conditions as dashed-line boxes. The arrows represent the transitions between states. The dashed arrow indicates that it starts from all health states. CMT, conventional medical treatment; HF, heart failure; ICD, implantable cardioverter-defibrillator.
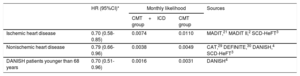
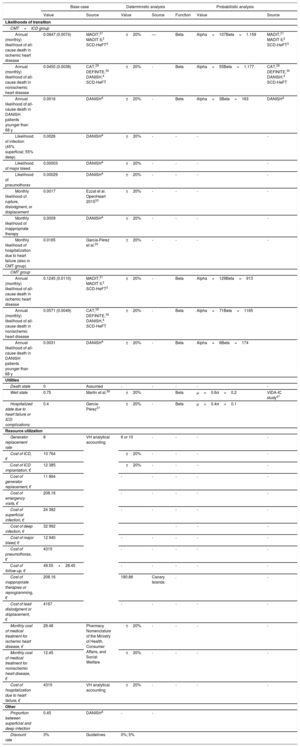
The likelihoods of death are shown for each group and each model in table 1. All likelihoods were obtained from a systematic review and meta-analysis on ICD efficacy in primary prevention (methodology described in supplementary data I), updating the search performed in a previous study20 up to May 30, 2018. Based on the meta-analysis, the efficacy data from the MADIT,21 MADIT II,2 and SCD-HeFT3 trials were used to estimate the likelihood of death among ischemic patients. However, the CABG-Patch,26 DINAMIT,27 and IRIS28 studies were excluded because they took place in the setting of coronary revascularization surgery or recent myocardial infarction. The likelihood of death among patients with nonischemic ventricular dysfunction was estimated using efficacy data from the CAT,29 DEFINITE,30 DANISH,4 and SCD-HeFT3 studies (placebo-controlled, except for the amiodarone treatment arm). The estimate calculation excluded the AMIOVIRT study,31 as it used amiodarone as a comparator, as well as the Pro-ICD study,32 which included patients wait-listed for a heart transplant. The COMPANION trial was also excluded because efficacy data could not be collected separately for patients with and without ischemic heart disease.
Baseline likelihoods of transition to all-cause death
| HR (95%CI)* | Monthly likelihood | Sources | ||
|---|---|---|---|---|
| CMT+ICD group | CMT group | |||
| Ischemic heart disease | 0.70 (0.58-0.85) | 0.0074 | 0.0110 | MADIT,21 MADIT II,2 SCD-HeFT3 |
| Nonischemic heart disease | 0.79 (0.66-0.96) | 0.0038 | 0.0049 | CAT,29 DEFINITE,30 DANISH,4 SCD-HeFT3 |
| DANISH patients younger than 68 years | 0.70 (0.51-0.96) | 0.0016 | 0.0031 | DANISH4 |
95%CI, 95% confidence interval; CAT, Cardiomyopathy Trial; CMT, conventional medical treatment; DANISH, DANish trial to assess efficacy of Implantable cardioverter defibrillators in patients with non-ischemic Systolic Heart failure; DEFINITE, Defibrillators in non-ischemic Cardiomyopathy Treatment Evaluation trial; HR, hazard ratio; ICD, implantable cardioverter defibrillator; MADIT, Multicenter Automatic Defibrillator Implantation Trial; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial.
The likelihoods were estimated from the cumulative rates of all randomized clinical trials included in both treatment groups, using the following formula:
The effect of ICD on the likelihood of all-cause death was assumed to be constant over the entire period, even though the randomized clinical trials had a mean follow-up period of 16 to 41 months (). To extrapolate mortality rates over the patient's entire lifetime, an age adjustment was performed using the 2017 mortality tables of the Spanish population published by the National Institute of Statistics (). All model parameters for the base case and for the sensitivity analyses are summarized in table 2.
Summary of the model parameters for the base case and sensitivity analyses
| Base case | Deterministic analysis | Probabilistic analysis | |||||
|---|---|---|---|---|---|---|---|
| Value | Source | Value | Source | Function | Value | Source | |
| Likelihoods of transition | |||||||
| CMT+ICD group | |||||||
| Annual (monthly) likelihood of all-cause death in ischemic heart disease | 0.0847 (0.0074) | MADIT,21 MADIT II,2 SCD-HeFT3 | ±20% | — | Beta | Alpha=107Beta=1.159 | MADIT,21 MADIT II,2 SCD-HeFT3 |
| Annual (monthly) likelihood of all-cause death in nonischemic heart disease | 0.0450 (0.0038) | CAT,29 DEFINITE,30 DANISH,4 SCD-HeFT | ±20% | - | Beta | Alpha=55Beta=1.177 | CAT,29 DEFINITE,30 DANISH,4 SCD-HeFT |
| Annual likelihood of all-cause death in DANISH patients younger than 68 y | 0.0016 | DANISH4 | ±20% | - | Beta | Alpha=3Beta=163 | DANISH4 |
| Likelihood of infection (45% superficial; 55% deep) | 0.0026 | DANISH4 | ±20% | - | - | - | - |
| Likelihood of major bleed | 0.00003 | DANISH4 | ±20% | - | - | - | - |
| Likelihood of pneumothorax | 0.00029 | DANISH4 | ±20% | - | - | - | - |
| Monthly likelihood of rupture, dislodgment, or displacement | 0.0017 | Ezzat et al. OpenHeart 201533 | ±20% | - | - | - | - |
| Monthly likelihood of inappropriate therapy | 0.0009 | DANISH4 | ±20% | - | - | - | - |
| Monthly likelihood of hospitalization due to heart failure (also in CMT group) | 0.0165 | García-Pérez et al.20 | ±20% | - | - | - | - |
| CMT group | |||||||
| Annual (monthly) likelihood of all-cause death in ischemic heart disease | 0.1245 (0.0110) | MADIT,21 MADIT II,2 SCD-HeFT3 | ±20% | - | Beta | Alpha=129Beta=913 | |
| Annual (monthly) likelihood of all-cause death in nonischemic heart disease | 0.0571 (0.0049) | CAT,29 DEFINITE,30 DANISH,4 SCD-HeFT | ±20% | - | Beta | Alpha=71Beta=1165 | |
| Annual likelihood of all-cause death in DANISH patients younger than 68 y | 0.0031 | DANISH4 | ±20% | - | Beta | Alpha=6Beta=174 | |
| Utilities | |||||||
| Death state | 0 | Assumed | - | - | - | - | - |
| Well state | 0.75 | Martín et al.36 | ±20% | Beta | μ=0.6σ=0.2 | VIDA-IC study47 | |
| Hospitalized state due to heart failure or ICD complications | 0.4 | García-Pérez37 | ±20% | - | Beta | μ=0.4σ=0.1 | |
| Resource utilization | |||||||
| Generator replacement rate | 8 | VH analytical accounting | 6 or 10 | - | - | - | - |
| Cost of ICD, € | 10 764 | ±20% | - | - | - | - | |
| Cost of ICD implantation, € | 12 385 | ±20% | - | - | - | - | |
| Cost of generator replacement, € | 11 864 | - | - | - | - | - | |
| Cost of emergency visits, € | 208.16 | - | - | - | - | - | |
| Cost of superficial infection, € | 24 382 | - | - | - | - | - | |
| Cost of deep infection, € | 32 992 | - | - | - | |||
| Cost of major bleed, € | 12 940 | - | - | - | - | - | |
| Cost of pneumothorax, € | 4315 | - | - | - | - | - | |
| Cost of follow-up, € | 49.55+28.45 | - | - | - | - | - | |
| Cost of inappropriate therapies or reprogramming, € | 208.16 | 180.86 | Canary Islands | . | - | ||
| Cost of lead dislodgment or displacement, € | 4167 | - | - | - | - | - | |
| Monthly cost of medical treatment for ischemic heart disease, € | 28.48 | Pharmacy Nomenclature of the Ministry of Health, Consumer Affairs, and Social Welfare | ±20% | - | - | - | - |
| Monthly cost of medical treatment for nonischemic heart disease, € | 12.45 | ±20% | - | - | - | - | |
| Cost of hospitalization due to heart failure, € | 4315 | VH analytical accounting | ±20% | - | - | - | - |
| Other | |||||||
| Proportion between superficial and deep infection | 0.45 | DANISH4 | - | - | |||
| Discount rate | 3% | Guidelines | 0%; 5% | ||||
CAT, Cardiomyopathy Trial; CMT, conventional medical treatment; DANISH, DANish trial to assess efficacy of Implantable cardioverter defibrillators in patients with non-ischemic Systolic Heart failure; DEFINITE, Defibrillators in non-ischemic Cardiomyopathy Treatment Evaluation trial; ICD, implantable cardioverter defibrillator; MADIT, Multicenter Automatic Defibrillator Implantation Trial; SCD-HeFT, Sudden Cardiac Death in Heart Failure Trial; VH, Vall d’Hebron Hospital.
The cost breakdown is listed in .
Perioperative complications (superficial or deep infection, major bleeds, pneumothorax) were differentiated from those potentially occurring throughout follow-up (malfunction due to rupture or dislodgment or inappropriate therapies).
Complication probabilities were obtained from the recent literature. Because registries and observational studies tend to underestimate complication rates,33 the perioperative complication rates and monthly rate of inappropriate therapies described in the DANISH trial were used, as this is the most recent randomized clinical trial and, therefore, the most representative of current clinical practice. The monthly probabilities of mechanical complications during follow-up (rupture, dislodgment, or displacement) were obtained from the articles by Ezzat et al.33 and Koneru et al.34
UtilitiesAll utilities were calculated using the same assumptions defined in the 2011 study.20 Utility is an index measuring preference-based quality of life from 0 to 1, where 0 represents death and 1 represents perfect health. It was assumed that 1 year of life with ventricular dysfunction was equal to 0.75 years with perfect health, based on previous studies.35,36 It was also assumed that, in the absence of complications, ICD usage did not affect quality of life. In health states involving patient worsening, quality of life was assumed to deteriorate by 0.35 points per year of life during the days the patient remained hospitalized.37
Resource use and costsResource use and costs were analyzed using direct health costs. In particular, the use of resources not directly ICD-related was presumed to be similar and not excessively high in either group and, therefore, these costs were not included. The regular drugs used in patients with heart failure with or without ischemic heart disease were included, assuming a percentage of use obtained from experts (clinical cardiologists and arrythmia specialists) in the Cardiology Department of Vall d’Hebron Hospital, as well as the target, maximum, or most common doses and the costs according to the Pharmaceutical Provision System of the Ministry of Health, Consumer Affairs, and Social Welfare ().
contains a description of the events defining each state, the assumptions made for each, the unit costs, and the sources of cost information. Resource use in the CMT+ICD group was based on routine clinical practice at Vall d’Hebron Hospital. The hospitalization costs for a first implant or complication-related replacement were estimated from a 22-patient sample provided by the analytical accounting at the same hospital. The unit costs include 20% overhead costs (eg, maintenance, services).
All costs are expressed as euros in 2018. When costs were not available for 2018, they were inflation-adjusted using the variation described by the National Institute of Statistics.
Sensitivity analysisTo evaluate the degree of uncertainty of the estimates, probabilistic sensitivity analyses were performed by assigning various distribution functions to the parameters: beta distribution for probabilities and utilities, and gamma-uniform distributions for resource use. The cost-effectiveness planes were obtained by Monte Carlo simulations of 10 000 iterations. The willingness-to-pay threshold for the base case was assumed to be the upper limit of the range of €20 000 to €25 000/QALY, empirically determined by Vallejo-Torres et al.38 Acceptability curves were also plotted to represent the probabilities of accepting ICD as a cost-effective alternative for different willingness-to-pay levels.39–41 To evaluate the impact on the result from the model estimations for each parameter, sensitivity analyses were performed using the variability found in the literature or variations of 20%. For intervention costs, the values used in the sensitivity analysis considered the variability between Vall d’Hebron Hospital and data provided by the Canary Islands Health Service. The base case assumes the most affordable device of those used at Vall d’Hebron Hospital and, therefore, this parameter was modified only with higher values in the sensitivity analysis. The results of this analysis were plotted in tornado charts.
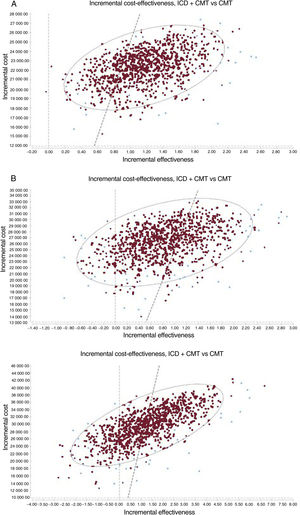
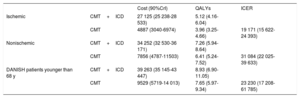
RESULTSTable 3 lists the results of the base case for the probabilistic analysis of the 3 scenarios analyzed. In the 3 cases, ICD implantation represents a QALY gain and a higher cost. The mean incremental cost-effectiveness ratios (ICERs) for CMT+ICD vs CMT were estimated at €19 171, €31 084, and €23 230/QALY for the base case in patients with ischemic heart disease, patients with nonischemic heart disease, and DANISH patients (with nonischemic heart disease) younger than 68 years, respectively. The points on the cost-effectiveness planes (figure 2) show the ICER result for each Monte Carlo simulation. Most points are in the upper right quadrant, indicating a higher cost and effectiveness for the CMT+ICD group compared with CMT in the 3 scenarios. In the nonischemic patient cohort, the percentage of cases to the left of the willingness-to-pay threshold of €25 000/QALY is higher. The probability that the CMT+ICD alternative would be cost-effective above a willingness-to-pay threshold of €25 000/QALY was 80% in patients with ischemic heart disease, 25% in nonischemic patients, and 55% in DANISH patients younger than 68 years ().
Base case results (probabilistic analysis), CMT+ICD vs CMT
| Cost (90%CrI) | QALYs | ICER | ||
|---|---|---|---|---|
| Ischemic | CMT+ICD | 27 125 (25 238-28 533) | 5.12 (4.16-6.04) | |
| CMT | 4887 (3040-6974) | 3.96 (3.25-4.66) | 19 171 (15 622-24 393) | |
| Nonischemic | CMT+ICD | 34 252 (32 530-36 171) | 7.26 (5.94-8.64) | |
| CMT | 7856 (4787-11503) | 6.41 (5.24-7.52) | 31 084 (22 025-39 633) | |
| DANISH patients younger than 68 y | CMT+ICD | 39 263 (35 145-43 447) | 8.93 (6.90-11.05) | |
| CMT | 9529 (5719-14 013) | 7.65 (5.97-9.34) | 23 230 (17 208-61 785) |
90%CrI, 90% credibility interval; CMT, conventional medical treatment; DANISH, DANish trial to assess efficacy of Implantable cardioverter defibrillators in patients with nonischemic Systolic Heart failure; ICD, implantable cardioverter defibrillator; ICER, incremental cost-effectiveness ratio; QALYs, quality-adjusted life years.
Incremental cost-effectiveness planes for ICD plus CMT vs CMT in patients with ischemic heart disease (A), patients with nonischemic heart disease (B), and DANISH patients younger than 68 years (C). The dashed line shows the threshold of €25 000/QALY. CMT, conventional medical treatment; ICD, implantable cardioverter-defibrillator; QALYs, quality-adjusted life years.
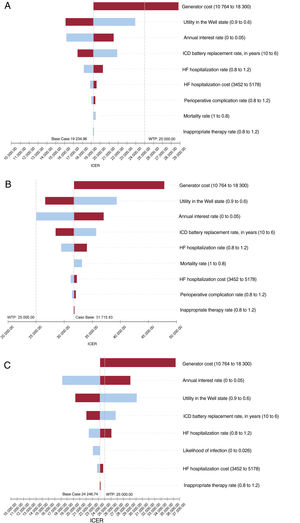
The sensitivity analyses (figure 3) show that the 3 models were sensitive, apart from the annual discount rate, device cost, utility value of the stable state (higher utility value in patients with stable heart failure signifies larger QALY gain if death is avoided by ICD use), and battery replacement rate (more replacements over a patient's lifetime will yield a higher cost per patient). In patients with ischemic heart disease, considering only device costs at the upper end of the range analyzed would lead to a different interpretation of the results compared with a threshold of €25 000/QALY. Two other important factors, but with a less relevant individual impact, would be to vary the cost of the hospitalization state (by reducing the number and/or length of hospitalizations) or to reduce the possibility of serious complications requiring repeat surgery and long hospital stays to negligible levels. In the case of patients with nonischemic heart disease, no single parameter change showed an interpretation economically favorable to the use of the ICD below a threshold of €25 000/QALY.
Results of univariate sensitivity analysis (tornado chart) in patients with ischemic heart disease (A), patients with nonischemic heart disease (B), and DANISH patients younger than 65 years (C). The middle value of the chart is the result for the base case, and the impact of an upward or downward parameter variation are shown to the right and left. The willingness-to-pay reference threshold is assumed to be €25 000/QALY. HF, heart failure; ICD, implantable cardioverter defibrillator; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; WTP, willingness to pay in €/QALY.
The present economic assessment from the perspective of the national health care system yielded similar results to those obtained in the last assessment in our setting carried out in 2011,19 but with differences explained by new data related to efficacy, resource use, and costs. An ICD implant for primary prevention in patients with ischemic heart disease yielded a slight gain in QALY (1.15 for a time horizon of 40 years) at a higher cost (€22 170), with an ICER of €19 278/QALY. In patients with ventricular dysfunction not due to ischemic heart disease, ICD implant represents a lower QALY gain (0.82) at a higher cost (€26 245), with an ICER of €32 006 /QALY, higher than the threshold of €25 000 /QALY. However, according to the DANISH trial data, the result for patients with nonischemic ventricular dysfunction would be more favorable for ICD in younger patients (younger than 68 years), in whom the benefit was somewhat higher (QALY difference, 1.22) and the ICER was near the threshold of €25 000 (€24 142 /QALY).
A willingness-to-pay threshold of €25 000/QALY38 was selected based on the only empirical study in our setting and accepted as a reference level by the Spanish Network of Agencies for Health Technology Assessment. However, the cost-effectiveness of ICD in patients with nonischemic ventricular dysfunction would be around the threshold of €30 000/QALY most commonly accepted until recently in Spain.
The differences compared with the 2011 evaluation were almost entirely due to differences in the incremental cost. The QALY gain was also small and very similar to that obtained in this study (0.95 in the ischemic subgroup; 0.79 in the nonischemic subgroup) whereas the estimated cost increase was higher, €36 376 in ischemic patients (ICER, €38 371) and €52 694 in nonischemic patients (ICER, €52 694). This cost difference is mainly attributable to the decrease in device cost and the longer mean life of the generators. This cost difference between ischemic and nonischemic patients is mainly attributable to differences in the likelihood of death due to higher survival of nonischemic patients and, therefore, a higher cumulative cost during follow-up.
In view of the ICER distribution in the patient subgroup with nonischemic ventricular dysfunction according to age, a high percentage of patients were unlikely to benefit from ICD, and decisions for these patients should be made understanding that the opportunity cost of implanting an ICD is high. Nevertheless, there is still some uncertainty regarding this patient profile, and efforts should focus on improving and encouraging individualized decisions. Several other prediction models based on other factors along with EF have been shown to have predictive value regarding the risk of sudden cardiac death, such as left ventricular end-diastolic diameter, ventricular tachycardia induction in the electrophysiologic study, the presence of spontaneous nonsustained ventricular tachycardia, left bundle-branch block, fragmented QRS,42 and particularly the presence, location, and extent of myocardial fibrosis.43 The health benefit obtained for each euro invested would be enhanced by encouraging the use of these or other models in differential risk prediction for sudden and nonsudden death to determine the patient profile most benefitting from prophylactic ICD implantation.
Likewise, it is important to consider patient preferences under various circumstances. For instance, this analysis did not include end-of-life replacements or ICD generator depletion with no discharges to the patient. In both cases, the need for replacement should be reassessed according to the patient's preferences and updated life expectancy.
LimitationsCost-utility estimation is based on a decision-making model, and hence its validity depends on the validity of the model's assumptions and parameters. Ultimately, the validity of the model depends on the sources of scientific evidence used to estimate the model parameters. The main aim of the present study was to include new evidence on efficacy and complication costs and rates that has become available since 2011. The clinical efficacy evidence was updated by using a systematic review and meta-analysis, incorporating the DANISH trial in the nonischemic ventricular dysfunction subgroup. The effectiveness evidence in patients with ischemic heart disease was the same as that used in 2011 and, therefore, may reflect a situation not entirely consistent with current clinical practice.
Likewise, resource use and costs are based on expert opinion, the health care process, and the analytical accounting of a single site, although we believe that the data are a more accurate picture of reality than those obtained from rates or from the literature. The external validity of the device cost estimate is more limited because the final fixed cost often depends on negotiations between the supply center and industry. Our approach chose the most affordable option (single-chamber generator from a specific supplier), but sensitivity analyses were carried out for other assumptions.
Although some patients received a dual-chamber device (14% according to the Spanish Cardiology Society registry), it was assumed that this was indicated less often for primary prevention.
Another important limitation is that ICD was not compared with ICD plus cardiac resynchronization therapy in patients with an indication to switch to this therapy due to heart failure progression. There is some debate about whether the expected benefits of ICD are greater in patients with no indication for CRT than in patients with an indication44 and, therefore, economic models should be developed with a structure that includes this possibility. However, this would require more sound data on efficacy and effectiveness, adverse events, and quality of life in these patients. Equally, we did not take into account the introduction of new alternative drug treatments, such as sacubitril-valsartan, despite proven efficacy and potentially higher cost-effectiveness than those of ICDs,45,46 possibly reducing the number of patients who may be eligible for an ICD. Follow-up also did not include long-term consequences that may have a different weight among patients with or without an ICD, such as the possibility of receiving a transplant or ventricular assist device.
CONCLUSIONSThe efficiency of the single-chamber ICD has improved in the past decade and is cost-effective in patients with ischemic and nonischemic left ventricular dysfunction younger than 68 years, assuming a willingness-to-pay threshold of €25 000/QALY. In older nonischemic patients, the ICER is around €30 000 /QALY.
- -
Compared with conventional medical treatment, ICD in primary prevention improves the survival of patients with ischemic and nonischemic heart failure with EF ≤ 35%.
- -
Prophylactic ICD use accounts for more than half of all indications, and is the fastest-growing indication in patients with dilated cardiomyopathy.
- -
A 2011 assessment found that ICD was cost-effective for primary prevention in patients with ischemic dilated cardiomyopathy who met the criteria for the MADIT trial, but not in patients who met the MADIT II criteria or had nonischemic dilated cardiomyopathy.
- -
Assuming a willingness-to-pay threshold of €25 000/QALY, ICD was is cost-effective for patients with ischemic left ventricular dysfunction.
- -
An ICD implant in patients with ventricular dysfunction not due to ischemic heart disease yields a lower QALY gain at a higher cost and would only be cost-effective below the threshold of €25 000 /QALY in younger patients (younger than 68 years), who obtain greater benefit.
This study was funded by the Ministry of Health, Consumer Affairs, and Social Welfare in the development framework of activities in the Annual Work Plan of Spanish Network of Health Technology Assessment and National Health Care Services (2018).
AUTHORS’ CONTRIBUTIONSA. Ribera, E. Giménez, and G. Oristrell prepared the first draft of the article. E. Giménez and J.R. Marsal performed the economic modeling. A. Ribera, J.R. Marsal, D. Osorio, and M. Ballesteros performed the meta-analysis. A. Ribera, J.R. Marsal, D. Osorio, M. Ballesteros, E. Ródenas, Y. Belahnech, and R. Escalona reviewed the abstracts and full articles to collect information for the systematic review. G. Oristrell, N. Rivas, I. Roca-Luque, and I. Ferreira-González participated as a clinical expert committee in determining the parameters of the economic model. L. García-Pérez developed the initial economic model and participated in its current adaptation. A. Ribera, I. Ferreira-González, and M. Espallargues sponsored the study and assisted with obtaining funding for its conduct. All authors contributed substantially to the interpretation of the results obtained in the systematic review and meta-analysis phase and in the financial modeling. All authors performed a critical review of the various draft versions and provided considerable intellectual input.
CONFLICTS OF INTERESTN. Rivas received fees from Abbott and Boston-Scientific unrelated to the article submitted. I. Roca-Luque received fees from Abbott, Medtronic, and Boston unrelated to the article submitted. E. Ródenas received an unconditional grant from Biotronik unrelated to the article submitted.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.05.004