The prevalence of heart failure (HF) has risen in parallel with improved survival in patients after a myocardial infarction and an aging population worldwide. In recent years, new percutaneous therapies have been developed to complement current established treatments for acute/decompensated and chronic HF and minimize risks. In acute presentations, the failure of medical treatment is no longer the end of the road in refractory circulatory shock; the use of mechanical circulatory support devices may be the next milestone in well-resourced health settings. Although evidence in this area is difficult to generate, research networks can facilitate the volume and quality of data needed to further augment the clinician's knowledge. Pulsatile (intra-aortic balloon pump), axial continuous (Impella), or centrifugal continuous pumps (TandemHeart; HeartMate PHP) together with percutaneously implanted extracorporeal membrane oxygenation are radically changing the prognosis of acute HF. Newer percutaneous therapies for chronic HF are based on attractive hypotheses, including left atrial decompression with shunting devices, left ventricle restoration through partitioning devices, or pressure-guided implantable therapies that may help to promptly treat decompensations. To date, only the last has been proved effective in a randomized study. Therefore, thorough research is still needed in this dynamic and promising field.
Keywords
The prevalence of heart failure (HF) has risen to 2% in adults in parallel with its progressive increase during the life course.1 World population aging has led to a growing global incidence of HF conditions, impairing patient wellbeing and increasing health care costs.1,2 Several improvements in the treatment of patients with HF with reduced ejection fraction have reduced the mortality rate, which continues to be higher than that of acute myocardial infarction (AMI), and the condition is associated with a rehospitalization rate of 40% per year.1,2 Hence, the mid-term prognosis of HF is still dire and in many scenarios is similar to that of cancer.2 In addition, HF represents between 1.8% and 3.1% of the public health budget in our environment, mostly due to in-hospital costs (73% in Spain).2
Therefore, efforts should focus on reducing the incidence of HF and decompensations, to avoid hospitalizations, and on improving prognosis. Several pharmacological and nonpharmacological strategies, including defibrillators, cardiac resynchronization therapy, left ventricular (LV) assist devices (LVAD), and heart transplant, represent important milestones in the treatment of advanced HF.1,3,4 In recent years, new percutaneous therapies have been developed to simplify and reduce the aggressiveness of interventions to complement current established treatments for acute/decompensated and chronic HF. In this review, we aim to summarize the main current and developing percutaneous alternatives for the treatment of both acute and chronic HF.
PERCUTANEOUS THERAPIES FOR ACUTE HEART FAILURECardiogenic shock is defined as a systemic tissular hypoperfusion secondary to low cardiac output despite adequate circulatory volume and LV filling pressure. As a consequence, mean blood pressure persists below 90mmHg or decreases by 30mmHg or more, with a cardiac index below 1.8 L/min/m2 (without hemodynamic support) or below 2.2 L/min/m2 if the patient is under support, and a wedge pulmonary pressure ≥ 15mmHg.5–8
The mortality rate in this serious scenario has improved in the last few decades but continues to be unacceptably high (≈ 50%), as does morbidity from the resultant multiple organ failure. The failure of inotropic and vasoactive drugs to overcome this critical status is no longer the end of the road in refractory circulatory shock; the use of percutaneous mechanical circulatory support (MCS) devices may be the next milestone in well-resourced health settings. The aim of MCS is two-fold: to stabilize the patient and to evaluate the subsequent options that can range from bridge to decision, to recovery, to long-term support devices such as percutaneous ventricular assist devices or to total artificial hearts, and/or heart transplant.5 The choice of the initial rescue MCS strategy has a significant bearing not only on limiting further iatrogenic harm in the acute setting, but also on planning long-term strategies in the absence of myocardial recovery. The development of predetermined institutional pathways is critical to the success of such MCS programs. Indeed, the ideal timing for percutaneous MCS device implantation remains controversial and the only general consensus is that they should be implanted sooner rather than later. In advanced HF, the INTERMACS score, which includes 7 categories, helps to better identify those stages (1 and 2) that may benefit from MCS due to failed inotropics therapy.9,10 Expert consensus is that percutaneous ventricular assist devices are indicated in patients undergoing high-risk coronary angioplasty (class IIB) and in those with cardiogenic shock due to AMI (class IB),5,11 although their potential use in cardiogenic shock due to other etiologies is also recognized ().
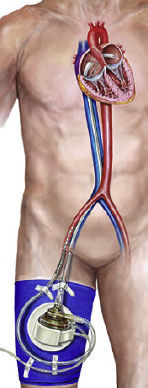
Although evidence in this area is difficult to generate, research networks can facilitate the volume and quality of data needed to further augment the clinician's knowledge of when, where, and which type of these technologies could and should be used. Mechanical circulatory support devices are classified according to the type of blood pump: Pulsatile (intra-aortic balloon pump [IABP]), axial continuous (Impella), or centrifugal continuous (TandemHeart; CentriMag; Rotaflow; HeartMate PHP).12 The IABP remains the most widely used method for mechanical assistance5 in patients experiencing LV failure, because of its moderate hemodynamic capabilities, prompt time to therapy, and relatively low complication rates. Percutaneous LVADs (pLVADs) represent an emerging option for partial or total circulatory support and several studies have compared the safety and efficacy of these devices with those of IABP. A description of each technique is provided below and summarized in Table 1.
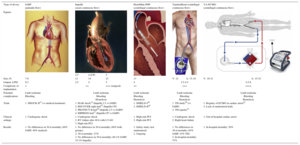
Technical Characteristics and Main Studies of Current Percutaneous Mechanical Circulatory Support Devices
| Type of device | IABP (pulsatile flow) | Impella (axial continuous flow) | HeartMate PHP (centrifugal continuous flow) | TandemHeart (centrifugal continuous flow) | VA-ECMO (centrifugal continuous flow) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Figures | |||||||||
| 2.5 | 4 (CP) | 5 | |||||||
| Size, Fr | 7-8 | 12 | 14 | 21 | 13 | V- 21 | A- 15-19 | V- 18-31 | A- 15-23 |
| Output, LPM | 0.5 | 2.5 | 4 | 5 | 4 | 3.5-4.5 | 4.5-8 | ||
| Complexity of implantation | + | ++ | +++ (surgical) | ++ | +++ | ++++ | |||
| Potential complications | Limb ischemia Bleeding | Limb ischemia Bleeding Hemolysis | Limb ischemia Bleeding Hemolysis | Limb ischemia Bleeding Hemolysis | Limb ischemia Bleeding Hemolysis | ||||
| Trials | 1. SHOCK-II13 (vs medical treatment) | 1. ISAR shock14 (Impella 2.5 vs IABP) 2. RECOVER right trial15 (Impella CP) 3. PROTECT-II trial16 (Impella 2.5 vs IABP) 4. IMPRESS trial17 (Impella CP vs IABP) | 1. SHIELD-I18 2. SHIELD-II19 | 1. TH study20 (vs IABP) 2. TH registry21 | 1. Registry of ECMO in cardiac arrest22 2. Lack of randomized trials | ||||
| Clinical settings | 1. Cardiogenic shock | 1. Cardiogenic shock 2. RV failure after 48 h with LVAD 3. High-risk PCI | 1. High-risk PCI 2. High-risk PCI | 1. Cardiogenic shock 2. Right heart failure | 1. Out-of-hospital cardiac arrest | ||||
| Results | 1. No differences in 30-d mortality (48% IABP; 49% medical) | 1. No difference in 30-d mortality (46% both groups) 2. 30-d mortality: 27% 3. No differences in 30-d mortality (40.1% IABP; 35.1% Impella) | 1. Safety study (not randomized) 2. Ongoing | 1. No differences in 30-d mortality (45% IABP; 43% TH) 2. In-hospital mortality: 57% | 1. In-hospital mortality: 50% | ||||
A, arterial; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LPM, liters per minute; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention; RV, right ventricle; TH, TandemHeart; V, venous; 30-d, 30-day.
Images reproduced with permission from the copyright owner.
Intra-aortic balloon pump is still the most widely used percutaneous ventricular assist device worldwide even though there is no proven evidence of its clinical benefit.13 Nevertheless, American and European guidelines recommend its use in patients with AMI and shock (class IIA),23,24 and in 2014 half of the patient receiving a heart transplant in Spain were under hemodynamic support with IABP.25,26 The main components include a 7.5 to 8.0 Fr dual-lumen catheter and a polystyrene balloon inflated with helium that allows rapid inflation and deflation due to its low viscosity. This creates a pulsatile pumping action with the deflation occurring during systole, which helps to reduce the afterload, increasing coronary perfusion and decreasing oxygen demand by the myocardium, but with a modest increase in cardiac output.27 Therefore, below a certain threshold of cardiac output, it may not be effective. In addition, its use is precluded by electrical instability, vascular disease, or the presence of aortic regurgitation.
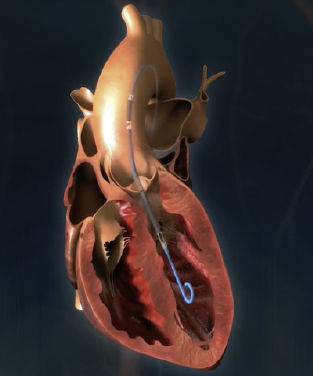
Impella (Abiomed, Danvers, Massachusetts, United States)A few randomized controlled trials have demonstrated better hemodynamic profiles for pLVADs compared with IABP.16,14,28 However, this has not translated into improved 30-day survival. Moreover, patients treated with pLVADs tended to have a higher incidence of leg ischemia and device-related bleeding.29 The use of positive inotropic drugs or vasopressors was expected to be lower in patients with Impella but no differences have been detected in the overall use of these agents. However, the use of Impella devices may increase in patients not responding to inotropes and IABP. Therefore, the decision-making process on treatment requires an integrated stepwise approach. In 2012 O’Neill et al.16 reported the results of the PROTECT II study, a prospective, randomized trial comparing hemodynamic support with Impella 2.5 vs IABP in individuals undergoing high-risk percutaneous coronary intervention. The trial was discontinued prematurely due to futility. Currently, there is also limited evidence on whether Impella improves the net health outcome in individuals with cardiogenic shock in the postcardiotomy setting or in high-risk percutaneous coronary interventions. Early study findings suggest that, although hemodynamic measures are consistently greater, clinically meaningful changes in outcomes have not been demonstrated.
Two percutaneous Impella devices exist: the Impella 2.5 and the Impella CP, both with a pigtail shape distally, which is introduced in the left ventricle, where a continuous flow is created toward the ascending aorta.30 Due to this mechanism, the flow does not require an activation signal–unlike the IABP–and is not affected by the presence of arrhythmias. Currently, it is approved for use for up to 4 (Food and Drug Administration [FDA]) or 5 days (Conformité Européenne [CE] mark).31 As limitations, it requires an adequately functioning right ventricle or the use of a right ventricular assist device, and its use is contraindicated by the presence of mechanical aortic prostheses or severe vascular disease. Complications are also similar to those of IABP, including bleeding events related to the need for systemic anticoagulation, and vascular injury. More specifically, inadvertent malpositioning of the device is a particular concern that may lead to rapid deterioration, and hemolysis may occur in up to 10% of patients.30–32 Both issues can be solved, in general, by repositioning and pump impeller adjustment.
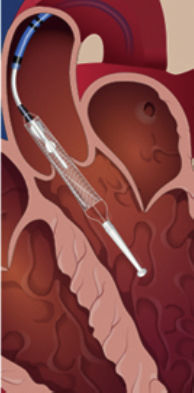
TandemHeart (CardiacAssist Inc, Pittsburgh, Pennsylvania, United States)This device has a 21-Fr cannula allocated in the left atrium (LA) through transeptal puncture impelling blood from the LA to the iliofemoral artery through a 15 to 19-Fr cannula.30 Its use has been approved by the FDA for 6hours whereas a CE mark has been obtained for up to 30 days.
Like the Impella device, right ventricular function should be preserved30 and vascular disease is a contraindication. There is limited information concerning its use in patients with aortic regurgitation or mechanical complications related to AMI.33,34 A specific source of complications is the need for transeptal pucture that should be guided by transesophageal echocardiography to minimize the risk.5 In addition, potential displacement of the cannula allocated in the LA to the right atrium is an unfrequent but challenging event. Several studies comparing TandemHeart to IABP revealed that the cardiac index rose in both groups (and in general hemodynamics improved), but was significantly higher in the TandemHeart group.35,36 In contrast, overall mortality at 30 days was similar in both groups, whereas the rate of events such as leg ischemia, severe bleeding, or sepsis was higher among TandemHeart participants. As before, the small number of patients probably did not allow for a meaningful evaluation of potential mortality differences but the results suggest that TandemHeart should only be used if the patient's hemodynamic status is not sufficiently improved by IABP.
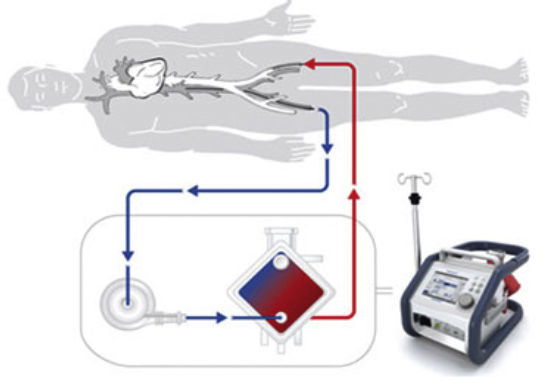
Extracorporeal Membrane OxygenationRecently, there has been increasing application of extracorporeal membrane oxygenation (ECMO) technology to provide MCS in an incremental fashion either as peripheral or central venoarterial ECMO or venovenous assist devices. The use of ECMO in cardiopulmonary resuscitation is also expanding with experienced centers reporting favorable outcomes.37,38 The percutaneous approach has been proved to be safe, effective and time saving, while minimizing bleeding from the cannula insertion site. Moreover, ECMO can be implanted at the bedside without the need for fluoroscopy guidance.5,30 However, percutaneous cannulation remains a challenge and is not free of complications. Indeed, ischemia of the corresponding extremity still requires surgical revision in 17% of patients. Therefore, a careful insertion technique, close surveillance, and monitoring are essential. The size of the cannula is similar to that of TandemHeart and portable devices are relatively simple to implant. The most frequent complications are related to thrombosis, bleeding,39 and infective events. As with TandemHeart, an antegrade perfusion cannula is recommended to prevent limb ischemia. The FDA and CE mark have approved the continuous use of the ECMO for 30 days, but longer duration has been consistently reported by the registry of the Extracorporeal Life Support Organization.22
There is a lack of randomized trials but the consensus is that ECMO can be useful in cases of cardiogenic shock, postcadiotomy failure, myocarditis, during resuscitation,37,38 or if there is primary graft failure in heart transplants. Indeed, one third of patients who underwent emergent heart transplant in Spain in 2014 were under ECMO.14
One of the main reasons for increased morbidity and mortality in patients under MCS is right ventricular failure. Currently, the use of venoarterial ECMO is probably the best alternative if this complication occurs, although the results of the RECOVER RIGHT study suggest a new potential and interesting use for Impella in this setting.15,38
Newer Percutaneous Therapies for Acute Heart FailureThe HeartMate PHP (Percutaneous Heart Pump) from Thoratec (St. Jude) is a new continuous axial flow catheter whose insertion is similar to that of the Impella device (Table 1) and with CE mark approval since July 2015 for its use in high-risk percutaneous coronary interventions. Its potential use in cardiogenic shock will be evaluated by the SHIELD-II study (coronary interventionS in HIgh-risk patiEnts using a novel percutaneous LV support device). Theoretically, it may diminish the rate of hemolysis through a reduction in shear stress and increase the stability within the left ventricle.
Several promising new technologies in preclinical research stages are also of interest in this context. This is a highly dynamic field and 2 examples that represent only the tip of the iceberg are the Aortix (Procyrion, Houston, Texas, United States) and the the BoLetz micro-LVAD (OCR, Yale, United Kingdom). The first consists of a self-expanding frame that fixes the pump to the aortic wall within the descending aorta, avoiding the risk of emboli to the supra-aortic vessels; following implantation, the 18-Fr catheter is removed, leaving only a flexible electrical power wire which is tunneled to a transcutaneous energy transfer system. The endovascularly deployable BoLetz micro-LVAD is a new concept in the early stages of development, which may represent the future concept of pLVAD destination therapies.
PERCUTANEOUS THERAPIES FOR CHRONIC HEART FAILUREOne of every 5 people will suffer from HF during their lifetime.1,2 One of the main reasons for this is the current better survival after an AMI, which has led to a higher prevalence of chronic LV dysfunction. However, HF still carries a poor prognosis and, in particular, progressive HF represents the main cause of death in patients in New York Heart Association (NYHA) class III or IV. Therefore, limiting this progression is a major target to improve not only quality of life but also mid-term survival.1 In addition, although there have been major developments in therapies for chronic HF with reduced ejection fraction, very few advances have been made in the heterogeneous scenario of HF with preserved ejection fraction, which lacks therapies with proven effectiveness, despite its comparable rates of morbidity and mortality.1 Given the large number of patients affected by this problem and the huge costs for the health system, it is not surprising that prolific research has been performed in this field in recent decades.40 The main new percutaneous strategies for the treatment of chronic HF are schematically depicted in Table 2. Percutaneous valvular therapies, as well as those aimed to treat mechanical complications, are not the object of this review.
New Percutaneous Therapies for the Treatment of Chronic Heart Failure
| Therapeutic strategies | LA decompression | LV restoration | Pressure-monitoring | Injectable therapies | Others | ||
|---|---|---|---|---|---|---|---|
| Devices | AFR (Mia Medical, Istanbul, Turkey) | V-Wave (V-Wave Ltd, Or-Akiva, Israel) | IASD (DC Devices Inc, Tewksbury, Massachusetts, United States) | Parachute (CardioKinetix Inc, Menlo Park, California, United States) | CardioMEMS HF (CardioMEMS, Atlanta, Georgia, United States) | Cell/Gene therapy + Helix transendocardial delivery system (BioCardia, San Carlos, California, United States) | New resynchronization devices, Neuromodulation, etc. |
| Figures | NA | ||||||
| Size, Fr | 14 | 14 | 16 | 14-16 | 12 | Variable | - |
| Complexity of implantation | ++ | ++++ | ++ | +++ | +++ | ||
| Trials/studies | Registry (PHT) | Registry (HFrEF)41 | Registry (HFpEF)42 | 1. Registries43 2. PARACHUTE-IV44 (trial ongoing) | 1. Registries45 2. CHAMPION trial46 | Registries | Registries |
| Results | Reduction in rehospitalizations | LV volume reduction and 6MWT distance improvement | Reduction in rehospitalizations. Included in HF guidelines | Under evaluation | Under evaluation | ||
| Other devices | “Home-made” devices | VenTouch (Mardil Medical, Minneapolis, Minnesota, United States) | Chronicle (Medtronic, Minneapolis, Minnesota, United States) | Algisyl (LoneStar Heart Inc, Laguna Hills, California, United States) | Multiple | ||
6MWT, 6-minute walk test; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LV, left ventricular; NA, not available; PHT, pulmonary hypertension.
Images reproduced with permission from the copyright owner.
Most HF patients have symtoms related to congestion and to low cardiac output. New therapies focus mainly on reducing left chamber filling pressures to improve congestion and decrease myocardial oxygen consumption.45,47 With this aim, different mechanisms have been proposed, including pressure-monitoring implantable devices, left atrial decompression, and LV reshaping systems.
Pressure-monitoring DevicesFor decades now, we have been using direct intracardiac pressure measurements through a Swan-Ganz catheter to obtain accurate diagnosis, evaluate prognosis, and treat patients with HF, based on the findings of classic retrospective studies. However, prospective studies including SUPPORT,48 ESCAPE,49 or PAC-Man,50 have failed to demonstrate its benefits but invasive pressure monitoring is still a commonly used tool in the management of HF patients.51,52 The missing gap between clinical practice and current evidence may be met by implantable pressure-monitoring devices. This new technology aims to prevent and promptly treat decompensations in chronic HF, through intracardiac wireless devices that can register LV filling pressures.53 The Chronicle device (Medtronic, Minneapolis, Minnesota, United States) makes a continuous recording of this pressure through a wire similar to pacemaker leads, within the right ventricular outflow tract. The COMPASS-HF study54 demonstrated that clinical decompensation was preceded by an increase in filling pressures, and helped to achieve a 36% reduction in the relative risk of rehospitalization in patients with chronic HF in NYHA class III. Similar findings were obtained from the REDUCE HR study,55 which was, however, discontinued prematurely due to a wire malfunctioning. For this reason, the FDA refused to approve its clinical use in 2007 but it nevertheless represents the proof of concept for the strategy of wireless pressure monitoring.
More recently, the CHAMPION study,45,46 which included HF patients in NYHA class III randomly assigned to conventional or pressure-guided treatment, reported that the CardioMEMS HF device (CardioMEMS, Atlanta, Georgia, United States) achieved a 28% reduction in the rehospitalization rate within the first 6 months following implantation. This device has been approved by the regulatory authorities, leading to its inclusion in the latest clinical guidelines for patients with HF.1 This small monitor (15×3mm) has a silicon encapsulation and nitinol anchoring to avoid its migration. Implantation is performed through a 12-Fr catheter advanced to the pulmonary artery that can be charged through radiofrecuency.
Finally, the HeartPOD (St. Jude Medical) monitors LA pressure through a 3×7mm monitor anchored in the interatrial septum. The ongoning LAPTOT-HF and CRT-D LAP studies56 aim to evaluate LA pressures and subsequently inform the patient on how to titer the medications accordingly. The main limitation of this new device may be the need for interatrial septal puncture and its associated risks but the risk/benefit ratio is still under evaluation.
Left Atrial Decompression Shunting DevicesThe rationale for shunting blood between atria as a means to reduce elevated pressures is based on medical knowledge from the 1960s (creation of atrial septostomies) to divert blood from one atrium to the other in the treatment of elevated pressures (both right and left off-loading).57 Moreover, currently the use of septostomy procedures is recommended by pulmonary hypertension guidelines for patients with advanced pulmonary hypertension with elevated right atrial pressure, and specific devices such as the Heartx AFR have been designed to this end (Table 2). Published data and feasibility studies in HF animal models demonstrate that shunting of 1000 to 1500mL/min for a 6mmHg pressure gradient (between left and right arterial pressure) and 1500 to 1900mL/min for a 10mmHg pressure gradient is expected to reduce LAP by 20% and 30%, respectively, without significantly increasing right atrial pressure.58,59 The population for which these new shunting devices are indicated includes patients with clinical sequels due to high LA pressure, representing approximately 90% of the patients hospitalized for pulmonary congestion.47 The reduction of this pressure below 18mmHg could potentially improve the patient's lung congestive state with a direct and positive effect on ventilation, dyspnea, and hospitalization rates.41,42,60,61
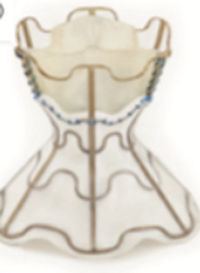
Two small series have reported the use of specific left-to-right shunting devices in humans to date. The first represents initial experience with the V-Wave device (V-Wave Ltd, Or Akiva, Israel) for the treatment of HF with reduced ejection fraction.41 The V-Wave device is a dedicated device, designed for percutaneous implantation in the fossa ovalis. This device attempts to address some of the limitations characterizing atrial septostomies. It is composed of a self-expandable hour-glass shaped nitinol shunt, with an expanded polytetrafluoroethylene encapsulation and 3 porcine pericardial leaflets sutured together. The V-Wave delivery catheter is a 14-Fr catheter intended to deliver the crimped shunt. Oral anticoagulation is recommmended for 3 months afterward. The device was successfully implanted in 10 patients; no device-related or procedural adverse events occurred during follow-up. The NYHA class improved from III to II-I in 89% of the patients, with a significant increase in quatily of life according to specific questionnaires and in the 6-minute walk test distance. Pulmonary capillary wedge pressure was reduced from 23 ± 5mmHg to 17 ± 8mmHg at 3 months, with no changes in right atrial pressure, pulmonary arterial pressure, or pulmonary resistance. No patient was admitted to hospital for worsening HF.41
In the second series, 68 patients with a diagonosis of HF with preserved ejection fraction were treated with the IASD (Corvia Medical, Tewksbury, Massachusetts, United States) with successful placement in 64 patients.42 The IASD is also a dedicated device composed of nitinol with an outer and inner diameter of 19mm and 8mm, respectively. Thus, a permanent 8-mm atrial septal defect is created. The legs of the device are flat on the LA side to minimize the risk of thrombus formation. A 16-Fr sheath is placed in the femoral vein and a proprietary delivery catheter is used to deliver the implant to the desired location at the fossa ovalis. After the procedure, patients were treated with dual antiplatelet therapy but no anticoagulation was recommended as there is no valve sutured inside the nitinol structure. No patient had periprocedural cardiac or cerebrovascular events or required a cardiac surgical intervention for device-related complications during 6 months of follow-up. A total of 52% had a reduction in pulmonary capillary wedge pressure at rest and 58% during exertion, with an increase in the mean exercise duration, which suggests that atrial blood shunting could be a new strategy for the management of HF with preserved ejection fraction.42
To date, the effectiveness of LA decompression shunting devices has not been compared with the standard of care in a randomized controlled trial. Therefore, this intriguing hypothesis and preliminary data on hemodynamic and functional improvement need to be interpreted with caution.
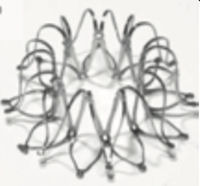
Left Ventricular Restoration Percutaneous DevicesThe current aggressive invasive strategies for AMI have increased survival rates. However, this has created a growing population with large residual myocardial injury. Left ventricular dilation and remodeling postmyocardial infarction is a well-known phenomenon driven by the reduction in contractility that increases wall stress and promotes the molecular activation of the fibrotic process leading to adverse remodeling. The concept of percutaneous ventricular restoration of the left ventricle, is based on the premise that a dedicated partitioning compliant device delivered via a catheter-based approach may achieve LV volume reduction, geometric reconfiguration, and synchronized wall motion to achieve a more effective ejection while minimizing the risk of a surgical approach. Its therapeutic efficacy depends on improvements in LV volumes reducing the wall stress.
The Parachute system (CardioKinetix Inc, Menlo Park, California, United States) includes a delivery system with a balloon that facilitates expansion of the device, and a preshaped delivery catheter. The device, with alternative sizes, comprises a self-expanding nitinol frame, an expanded polytetrafluoroethylene impermeable membrane with an atraumatic radiopaque polymer foot aimed to provide a contact point between the LV apical wall, which allows the device to be positioned with a vector toward the outflow tract.
After initial promising preclinical results,62 a prospective nonrandomized study (PARACHUTE III) aimed to assess the long-term safety and efficacy of the Parachute device. One hundred patients with NYHA class II-IV ischemic HF and LV ejection fraction between 15% and 40% with dilated akinetic or dyskinetic anterior-apical wall were enrolled. Of the 100 enrolled patients, device implantation was successful in 97 participants. The primary safety endpoint was procedural-related major adverse cardiac/cerebral events (7%) and the secondary safety endpoint was the composite of mortality and morbidity (32.3%). Secondary efficacy endpoints included several imaging and functional parameters and were successful in terms of LV volume reduction and improvement in 6-minute walk distance.43 This acceptable safety and efficacy result also needs to be validated in randomized controlled trials, such as the ongoing PARACHUTE IV.44
In summary, these novel therapies aiming to improve the symptoms and prognosis of patients with chronic HF are promising, but there is still a gap in the evidence that will soon be narrowed by ongoing studies. The first step, demonstrating the importance of pressure-guided treatment of chronic HF through randomized analysis has already been taken and represents the first milestone for this new generation of percutaneous therapies.
FINAL COMMENTSHeart failure has a growing incidence and variable mechanisms that require specific therapies. In acute or decompensated presentations, the failure of medical treatment no longer means that therapeutic options have been exhausted in refractory circulatory shock; the use of MCS devices represents the next milestone in well-resourced health settings. Although evidence on survival is scarce because this particular scenario hinders randomization, the growing range of alternative devices must be handled with caution but also bravely in order to reduce the unacceptable mortality rate in patients with cardiogenic shock.
Even more complex is the management of patients with chronic HF. Because of the large number of individuals with this problem, the deterioration in their quality of life, and the health care-related costs, new and equally effective and efficient strategies are needed to improve the quality of life of our patients with a parallel reduction in hospitalizations and decompensations. The slow process of evaluation of new percutaneous therapies to treat chronic HF is both necessary and sufficient to integrate those technologies with demonstrated benefits in our therapeutic arsenal.
In conclusion, the armamentarium of new percutaneous devices for the treatment of acute and chronic HF is leading to a paradigm shift from a common therapeutic path to an individualized approach in the management of these patients. This dynamic field still requires thorough investigation and caution, but also open-minded physicians ready to move forward into this thrilling new era.
FUNDINGL.H. Varela-Falcón received support from the Fundación Carolina-BBVA. The ICICOR received support to a related project from the Instituto de Salud Carlos III (FIS Project PI 15/01695) and by ERDF funds.
CONFLICTS OF INTERESTI.J. Amat-Santos has received institutional grant from V-Wave Ltd. W.T. Abraham is consultant and shareholder of V-Wave Ltd and has been a consultant to and received grants from Medtronic Inc, CardioMEMS, St. Jude Medical, and CVRx.
To Amada Recio-Platero for her kind help and to the rest of the professionals of the Heart Failure Unit in the ICICOR (Instituto de Ciencias del Corazón).