Above and beyond the encouraging findings of recent clinical trials (RE-LY, ROCKET-AF, ARISTOTLE y ENGAGE AF-TIMI 48),1–4 which are discussed below, the arrival of the new oral anticoagulants (NOAGs) represents an improvement compared with standard treatment (vitamin K antagonists such as warfarin and acenocoumarol) in the prevention of thromboembolic complications in patients with nonvalvular atrial fibrillation (AF).
The NOAGs (dabigatran, rivaroxaban, and apixaban are currently available in Spain and edoxaban will probably receive approval) overcome many of the drawbacks traditionally associated with vitamin K antagonists (narrow therapeutic window, variable response, multiple interactions with food and other drugs, and slow onset and offset). The most immediate and obvious consequences of these limitations are the need for regular monitoring and continuous dose adjustments, in addition to the dietary restrictions, and scrupulous care when prescribing concomitant medication.5 As a result, patients’ quality of life has been greatly limited. The most important consequence, however, is that many patients with AF and a clear indication for anticoagulation are not receiving any therapy.5,6 Moreover, even among patients taking vitamin K antagonists in Spain, approximately 35% to 40% have poorly controlled anticoagulation, in terms of the international normalized ratio (INR), with a major impact on the risk of both stroke and bleeding.5–8 The NOAGs, with their broad therapeutic window, predictable anticoagulant response, lack of dietary restrictions, and limited drug-drug interactions, enable a constant and predictable anticoagulation, thereby obviating the need for regular monitoring of anticoagulant response and constant dose adjustments.
If these advantages were not enough, the different clinical trials point to additional benefits that are clearly of high clinical relevance. A recent metaanalysis of the RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE AF-TIMI 48 studies found that, in comparison with warfarin, NOAGs significantly reduce the risk of stroke or systemic embolism (by 19%; P < .0001), all-cause death (by 10%; P = .0003), and intracranial bleeding (by 52%; P < .0001).9 In addition, NOAGs showed a trend towards a reduction in major bleeding (P = 0.06), an important benefit in subjects with worse INR control (time in therapeutic window < 66%).
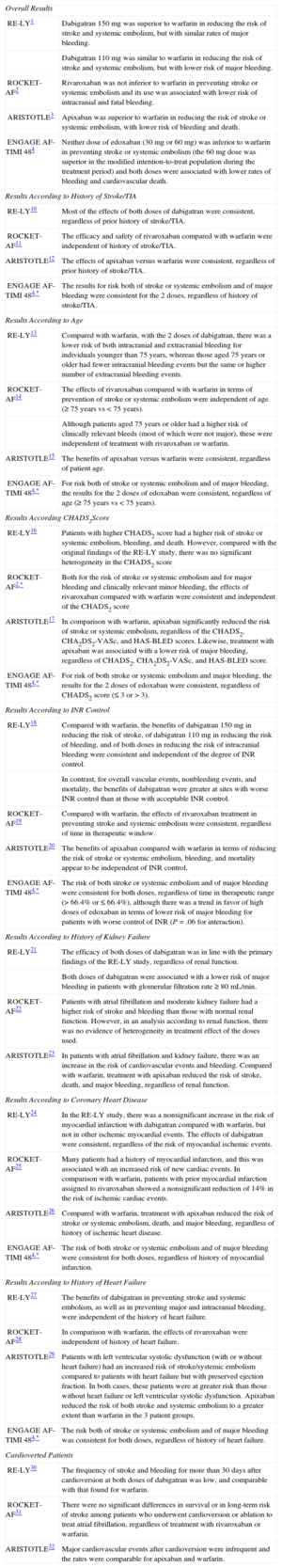
If the primary findings of the main clinical trials were not sufficiently strong evidence, in recent years substudies have further clarified the role of NOAGs in the treatment of patients with AF, particularly in specific situations. Table summarizes some of the most relevant substudies.1–4,10–32 Given that the primary results of the ENGAGE AF-TIMI 48 trial were published only recently, no substudies have been published as yet. The information on certain clinical situations has therefore been extracted from the supplementary material for the original publication (the same applies to some specific instances with the other NOAGs). In general, the efficacy and safety of rivaroxaban, apixaban, and edoxaban, as well as most of the effects of dabigatran, were consistent with the findings obtained in the original studies, regardless of whether patients had a history of stroke or transient ischemic accident.4,10–12 Moreover, although patients aged 75 years or older were at greater risk of bleeding, the benefits of NOAGs were age-independent.4,13–15 Likewise, the efficacy and safety of NOAGs were robust and independent of the CHADS2 score and history of kidney or heart failure.2,4,16,17,21,23,27–29 In terms of INR control, the benefits (both in reduction of stroke/systemic embolism and safety) of all the NOAGs were robust and independent of mean INR control in the participating centers. With respect to overall vascular events, nonbleeding events, and mortality, the benefits of dabigatran were greater at sites with worse INR control than at those with adequate INR control. There was also a trend in favor of high-dose edoxaban in terms of lower risk of major bleeding in patients with worse INR control.4,18–20 Although the efficacy and safety results were consistent regardless of the presence of history of coronary artery disease with apixaban and edoxaban, in the RE-LY study comparing dabigatran with warfarin there was a nonsignificant increase in the risk of myocardial infarction but not of other ischemic myocardial events. Thus, in general, dabigatran shows consistently positive effects in patients with and without a prior history of ischemic heart disease or myocardial infarction. In comparison with warfarin, patients with prior myocardial infarction assigned to rivaroxaban showed a nonsignificant reduction of 14% in the risk of ischemic cardiac events.4,24–26
Results of the Main Substudies of the RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE AF-TIMI 48 Clinical Trials
| Overall Results | |
| RE-LY1 | Dabigatran 150mg was superior to warfarin in reducing the risk of stroke and systemic embolism, but with similar rates of major bleeding. |
| Dabigatran 110mg was similar to warfarin in reducing the risk of stroke and systemic embolism, but with lower risk of major bleeding. | |
| ROCKET-AF2 | Rivaroxaban was not inferior to warfarin in preventing stroke or systemic embolism and its use was associated with lower risk of intracranial and fatal bleeding. |
| ARISTOTLE3 | Apixaban was superior to warfarin in reducing the risk of stroke or systemic embolism, with lower risk of bleeding and death. |
| ENGAGE AF-TIMI 484 | Neither dose of edoxaban (30mg or 60 mg) was inferior to warfarin in preventing stroke or systemic embolism (the 60mg dose was superior in the modified intention-to-treat population during the treatment period) and both doses were associated with lower rates of bleeding and cardiovascular death. |
| Results According to History of Stroke/TIA | |
| RE-LY10 | Most of the effects of both doses of dabigatran were consistent, regardless of prior history of stroke/TIA. |
| ROCKET-AF11 | The efficacy and safety of rivaroxaban compared with warfarin were independent of history of stroke/TIA. |
| ARISTOTLE12 | The effects of apixaban versus warfarin were consistent, regardless of prior history of stroke/TIA. |
| ENGAGE AF-TIMI 484,* | The results for risk both of stroke or systemic embolism and of major bleeding were consistent for the 2 doses, regardless of history of stroke/TIA. |
| Results According to Age | |
| RE-LY13 | Compared with warfarin, with the 2 doses of dabigatran, there was a lower risk of both intracranial and extracranial bleeding for individuals younger than 75 years, whereas those aged 75 years or older had fewer intracranial bleeding events but the same or higher number of extracranial bleeding events. |
| ROCKET-AF14 | The effects of rivaroxaban compared with warfarin in terms of prevention of stroke or systemic embolism were independent of age (≥ 75 years vs < 75 years). |
| Although patients aged 75 years or older had a higher risk of clinically relevant bleeds (most of which were not major), these were independent of treatment with rivaroxaban or warfarin. | |
| ARISTOTLE15 | The benefits of apixaban versus warfarin were consistent, regardless of patient age. |
| ENGAGE AF-TIMI 484,* | For risk both of stroke or systemic embolism and of major bleeding, the results for the 2 doses of edoxaban were consistent, regardless of age (≥ 75 years vs < 75 years). |
| Results According CHADS2Score | |
| RE-LY16 | Patients with higher CHADS2 score had a higher risk of stroke or systemic embolism, bleeding, and death. However, compared with the original findings of the RE-LY study, there was no significant heterogeneity in the CHADS2 score. |
| ROCKET-AF2,* | Both for the risk of stroke or systemic embolism and for major bleeding and clinically relevant minor bleeding, the effects of rivaroxaban compared with warfarin were consistent and independent of the CHADS2 score. |
| ARISTOTLE17 | In comparison with warfarin, apixaban significantly reduced the risk of stroke or systemic embolism, regardless of the CHADS2, CHA2DS2-VASc, and HAS-BLED scores. Likewise, treatment with apixaban was associated with a lower risk of major bleeding, regardless of CHADS2, CHA2DS2-VASc, and HAS-BLED score. |
| ENGAGE AF-TIMI 484,* | For risk of both stroke or systemic embolism and major bleeding, the results for the 2 doses of edoxaban were consistent, regardless of CHADS2 score (≤ 3 or > 3). |
| Results According to INR Control | |
| RE-LY18 | Compared with warfarin, the benefits of dabigatran 150mg in reducing the risk of stroke, of dabigatran 110mg in reducing the risk of bleeding, and of both doses in reducing the risk of intracranial bleeding were consistent and independent of the degree of INR control. |
| In contrast, for overall vascular events, nonbleeding events, and mortality, the benefits of dabigatran were greater at sites with worse INR control than at those with acceptable INR control. | |
| ROCKET-AF19 | Compared with warfarin, the effects of rivaroxaban treatment in preventing stroke and systemic embolism were consistent, regardless of time in therapeutic window. |
| ARISTOTLE20 | The benefits of apixaban compared with warfarin in terms of reducing the risk of stroke or systemic embolism, bleeding, and mortality appear to be independent of INR control. |
| ENGAGE AF-TIMI 484,* | The risk of both stroke or systemic embolism and of major bleeding were consistent for both doses, regardless of time in therapeutic range (> 66.4% or ≤ 66.4%), although there was a trend in favor of high doses of edoxaban in terms of lower risk of major bleeding for patients with worse control of INR (P=.06 for interaction). |
| Results According to History of Kidney Failure | |
| RE-LY21 | The efficacy of both doses of dabigatran was in line with the primary findings of the RE-LY study, regardless of renal function. |
| Both doses of dabigatran were associated with a lower risk of major bleeding in patients with glomerular filtration rate ≥ 80 mL/min. | |
| ROCKET-AF22 | Patients with atrial fibrillation and moderate kidney failure had a higher risk of stroke and bleeding than those with normal renal function. However, in an analysis according to renal function, there was no evidence of heterogeneity in treatment effect of the doses used. |
| ARISTOTLE23 | In patients with atrial fibrillation and kidney failure, there was an increase in the risk of cardiovascular events and bleeding. Compared with warfarin, treatment with apixaban reduced the risk of stroke, death, and major bleeding, regardless of renal function. |
| Results According to Coronary Heart Disease | |
| RE-LY24 | In the RE-LY study, there was a nonsignificant increase in the risk of myocardial infarction with dabigatran compared with warfarin, but not in other ischemic myocardial events. The effects of dabigatran were consistent, regardless of the risk of myocardial ischemic events. |
| ROCKET-AF25 | Many patients had a history of myocardial infarction, and this was associated with an increased risk of new cardiac events. In comparison with warfarin, patients with prior myocardial infarction assigned to rivaroxaban showed a nonsignificant reduction of 14% in the risk of ischemic cardiac events. |
| ARISTOTLE26 | Compared with warfarin, treatment with apixaban reduced the risk of stroke or systemic embolism, death, and major bleeding, regardless of history of ischemic heart disease. |
| ENGAGE AF-TIMI 484,* | The risk of both stroke or systemic embolism and of major bleeding were consistent for both doses, regardless of history of myocardial infarction. |
| Results According to History of Heart Failure | |
| RE-LY27 | The benefits of dabigatran in preventing stroke and systemic embolism, as well as in preventing major and intracranial bleeding, were independent of the history of heart failure. |
| ROCKET-AF28 | In comparison with warfarin, the effects of rivaroxaban were independent of history of heart failure. |
| ARISTOTLE29 | Patients with left ventricular systolic dysfunction (with or without heart failure) had an increased risk of stroke/systemic embolism compared to patients with heart failure but with preserved ejection fraction. In both cases, these patients were at greater risk than those without heart failure or left ventricular systolic dysfunction. Apixaban reduced the risk of both stroke and systemic embolism to a greater extent than warfarin in the 3 patient groups. |
| ENGAGE AF-TIMI 484,* | The risk both of stroke or systemic embolism and of major bleeding was consistent for both doses, regardless of history of heart failure. |
| Cardioverted Patients | |
| RE-LY30 | The frequency of stroke and bleeding for more than 30 days after cardioversion at both doses of dabigatran was low, and comparable with that found for warfarin. |
| ROCKET-AF31 | There were no significant differences in survival or in long-term risk of stroke among patients who underwent cardioversion or ablation to treat atrial fibrillation, regardless of treatment with rivaroxaban or warfarin. |
| ARISTOTLE32 | Major cardiovascular events after cardioversion were infrequent and the rates were comparable for apixaban and warfarin. |
INR, international normalized ratio; TIA, transient ischemic attack.
In short, we can affirm that the results of the new analyses are generally well aligned with the findings of the primary analyses, regardless of history of stroke, kidney failure, ischemic heart disease, heart failure, age, CHADS2 score, and anticoagulation control. These substudies therefore further support the role of NOAGs in the prevention of thromboembolic complications in patients with nonvalvular AF.
The data from clinical trials, both in the main analyses and subsequent subanalyses, are robust, consistent, and congruent, but given the recent arrival of these drugs, their efficacy and safety in clinical practice are as yet not well known. Fortunately, an increasing number of studies in the clinical practice setting are being published; these studies confirm the efficacy and safety of NOAGs (with the results being even better than expected).33–36
The current strong evidence in support of NOAGs in general begs the question whether these agents are equivalent. If truth be told, the current data cannot provide a definitive answer to this question. The clinical trials compared a NOAG with warfarin; no head-to-head comparisons of NOAGs have been conducted.1–4 Moreover, the populations included in the studies were very different, with the exception of ARISTOTLE and RE-LY, which were very similar (if not identical). It is thus difficult to rigorously affirm that one NOAG is significantly better or worse than another. However, there are important differences between the NOAGs. For example, dabigatran and apixaban are administered twice a day, whereas rivaroxaban and edoxaban are taken once a day. The once-daily dosing may encourage treatment adherence, but a missed dose may be associated with greater risk of harmful consequences. The other fundamental difference between the NOAGs lies in their metabolism and elimination pathway. It seems evident that dabigatran is not the most appropriate choice in patients with creatinine clearance of approximately 30mL/min (particularly in the case of elderly patients, who are at greater risk of a sudden deterioration in renal function in the event of dehydration, etc.). However, if the choice of drug depends on determining maximum efficacy for reducing stroke and systemic embolism, dabigatran at a dose of 150mg/12hours would seem the best option. If the most favorable safety profile is sought, apixaban seems to be the best option, while rivaroxaban and edoxaban have the most convenient dosing. Dabigatran was the first NOAG to be launched, and so this agent is supported by the most extensive body of clinical experience; mean follow-up in published data exceeds 4 years.37 There is also a key unquantifiable factor when physicians choose which drug to prescribe: their experience and knowledge of the drug, which allows them to manage the dose and take into account possible interactions, complications, and any intervening situations.
Finally, the different cost-effectiveness studies of NOAGs, conducted in different countries and by different authors, are consistent among themselves. The overall conclusion is that for patients most at risk, whether of thromboembolic or bleeding complications, NOAGs would seem to be the most recommended option in patients who achieve poor anticoagulation control with vitamin K antagonists.38,39 However, despite all the evidence and the studies currently available, the market share of NOAGs is less than expected given the degree of INR control in Spain.5–7 Restrictive policies in certain autonomous regions, not based on scientific criteria with a view to medium- and long-term control of expenditure, but rather a reflection of short-term thinking (for the control of immediate drug expenditure), will probably turn out to be more expensive in the long run. These policies will also prevent access to these drugs for many patients who would truly benefit, with disastrous consequences both for the patients and families, as well as for society in general.
CONFLICTS OF INTERESTA. Martínez-Rubio has participated in several research projects and has received grants to participate in scientific steering committees for Bayer, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer.
The authors would like to thank Pablo Pons of Content Ed Net Madrid for his assistance in editing the manuscript.