Cardiac arrhythmias are relatively common in the pediatric age. The yearly estimated incidence of supraventricular tachycardia in persons younger than 19 years is 13 cases per 100 000 population.1 Currently, the safety and effectiveness of percutaneous ablation treatment in this population is considered comparable to that of adults undergoing cardiac ablation, although the number reported is considerably smaller. Most literature on pediatric cardiac ablation is from multicenter registries in the United States,2–4 where these patients are treated in referral units with a large volume of cases, exceeding 100 per year. This factor has been associated with better clinical outcomes.5 Nonetheless, some recent reports from European countries have shown similar effectiveness and safety results, even though the number of cases treated is significantly smaller.6,7
Regardless of the health care setting, ablation in pediatric patients, particularly those younger than 12 years, involves specific clinical characteristics, technical requisites, and management requirements. Hence, the professionals attending these patients should be well prepared to carry out the procedure with guaranteed safety and resolve any unforeseen complications that may arise.
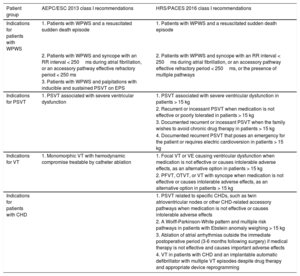
Treatment IndicationsThe guidelines for the diagnosis and treatment of arrhythmias in pediatric patients and those with congenital heart disease (CHD) from AEPC/EHRA in 20131 and PACES/HRS in 20168 provide specific information on the etiology, epidemiology, diagnosis, and treatment strategies for cardiac ablation in patients 0 to 18 years of age (Table).
Comparison of the Class I Indications for Ablation Between the European and American guidelines
| Patient group | AEPC/ESC 2013 class I recommendations | HRS/PACES 2016 class I recommendations |
|---|---|---|
| Indications for patients with WPWS | 1. Patients with WPWS and a resuscitated sudden death episode | 1. Patients with WPWS and a resuscitated sudden death episode |
| 2. Patients with WPWS and syncope with an RR interval < 250ms during atrial fibrillation, or an accessory pathway effective refractory period < 250 ms | 2. Patients with WPWS and syncope with an RR interval < 250ms during atrial fibrillation, or an accessory pathway effective refractory period < 250ms, or the presence of multiple pathways | |
| 3. Patients with WPWS and palpitations with inducible and sustained PSVT on EPS | ||
| Indications for PSVT | 1. PSVT associated with severe ventricular dysfunction | 1. PSVT associated with severe ventricular dysfunction in patients > 15 kg |
| 2. Recurrent or incessant PSVT when medication is not effective or poorly tolerated in patients > 15 kg | ||
| 3. Documented recurrent or incessant PSVT when the family wishes to avoid chronic drug therapy in patients > 15 kg | ||
| 4. Documented recurrent PSVT that poses an emergency for the patient or requires electric cardioversion in patients > 15 kg | ||
| Indications for VT | 1. Monomorphic VT with hemodynamic compromise treatable by catheter ablation | 1. Focal VT or VE causing ventricular dysfunction when medication is not effective or causes intolerable adverse effects, as an alternative option in patients > 15 kg |
| 2. PFVT, OTVT, or VT with syncope when medication is not effective or causes intolerable adverse effects, as an alternative option in patients > 15 kg | ||
| Indications for patients with CHD | 1. PSVT related to specific CHDs, such as twin atrioventricular nodes or other CHD-related accessory pathways when medication is not effective or causes intolerable adverse effects | |
| 2. A Wolff-Parkinson-White pattern and multiple risk pathways in patients with Ebstein anomaly weighing > 15 kg | ||
| 3. Ablation of atrial arrhythmias outside the immediate postoperative period (3-6 months following surgery) if medical therapy is not effective and causes important adverse effects | ||
| 4. VT in patients with CHD and an implantable automatic defibrillator with multiple VT episodes despite drug therapy and appropriate device reprogramming |
AEPC/ESC, Association for European Paediatric and Congenital Cardiology/European Society of Cardiology; CHD, congenital hear disease; EPS; electrophysiology study; HRS/PACES, Heart Rhythm Society/Pediatric and Congenital Electrophysiology Society; OTVT, outflow tract ventricular tachycardia; PFVT, posterior fascicular ventricular tachycardia; PSVT, paroxysmal supraventricular tachycardia; VE, ventricular extrasystoles; VT, ventricular tachycardia; WPWS; Wolff-Parkinson-White syndrome
Although these 2 guidelines are similar in general terms, there are some slight differences in the indications provided. For example, the class I indications for patients with Wolff-Parkinson-White syndrome: although both guidelines include as a class I indication the presence of this syndrome in patients with an episode of resuscitated sudden cardiac death and in patients with syncope and an RR interval < 250ms during atrial fibrillation (corresponding to a heart rate of 240 bpm) or an accessory conduction pathway effective refractory period < 250ms, the American guidelines also include syncope with more than 1 accessory pathway as a risk factor. Furthermore, the guidelines differ regarding the treatment of paroxysmal supraventricular tachycardia (PSVT) without pre-excitation in patients with no heart disease. In these cases, the AEPC/EHRA guidelines only include PSVT associated with severe ventricular dysfunction as a class I indication. In the HRS/PACES guidelines, apart from ventricular dysfunction, a class I indication is also given to cases of recurrent or incessant PSVT in which medication is ineffective or poorly tolerated in patients weighing more than 15kg, in patients with hemodynamic deterioration with syncope as a symptom associated with PSVT, and in cases with an electric cardioversion requirement for initial treatment in patients weighing more than 15kg. Lastly, family preference is also included as a class I indication in patients with weight higher than 15kg.
For the treatment of ventricular arrhythmias, the guidelines show no relevant differences. Both have a class I indication for patients with symptomatic ventricular tachycardia (VT) when drug therapy is ineffective in controlling the arrhythmia. The HRS/PACES guidelines also include a class I indication for intolerance to medication and for family preference as an alternative to medical therapy in patients weighing more than 15kg.
Specific indications for arrhythmia ablation treatment in patients with CHD are only provided in the American guidelines. These include a class I indication for patients with PSVT related to specific CHDs, such as twin atrioventricular nodes or other CHD-related accessory pathways when medication is not effective or produces intolerable adverse effects. A class I indication is also assigned to ablation of multiple accessory pathways in patients with Ebstein anomaly, and to ablation of atrial arrhythmia outside the immediate postoperative period (3-6 months following surgery) when medical treatment is not effective and produces important adverse effects. Finally, a class I recommendation is included for VT in CHD patients with an implantable automatic defibrillator experiencing several episodes of tachycardia despite drug therapy and adequate device reprogramming, with the aim of avoiding activation of multiple shocks.
Both guidelines include a specific mention regarding cardiac ablation in lactating infants and children younger than 5 years, as there is sufficient evidence to consider the patient's weight as an independent risk factor for severe complications, including some reported deaths.9 Up to now, the AEPC/EHRA guidelines have defined these as patients weighing < 15kg, but in the latest American guidelines this limit has been slightly modulated, with smaller patients being referred to as those weighing less than “approximately 15 kg”. Despite this slight difference in the definition, there is consensus that professionals should be more restrictive with the indications in this age group, and attempt to optimize medical therapy by including several drug combinations to delay cardiac ablation. In addition, the following measures are recommended: 1) that these procedures should be carried out by electrophysiologists with experience in pediatric patients; 2) that ablation should be performed with a tailored strategy that minimizes the number of applications; and 3) that cryoablation should be used before radiofrequency in substrates carrying an elevated risk of atrioventricular block. Finally, special mention is made of the subgroup of smallest patients, weighing between 3 and 7kg or younger than 6 months, in whom ablation should only be performed for life-threatening cardiac arrhythmia after failure of several combinations of antiarrhythmic drugs.
Effectiveness And Complications Of Pediatric Cardiac AblationData on the effectiveness of ablation mainly come from retrospective and prospective registries in the United States, which have shown a rise in the rate of effective ablations from 90.4% in the 1991 to 1996 period to 95.2% from 1996 to 1999.2,3 These results are very similar to those observed in a prospective registry including 2761 patients from 41 centers, reporting an overall effectiveness rate of 93%.4 To date, there are 2 population registries of pediatric ablation procedures in Europe, 1 in Finland6 and 1 in the Czech Republic,7 which show an overall final effectiveness rate similar to the values in the American registries, specifically 91% and 96%, respectively. Recent nonpopulation studies have reported very high effectiveness (> 98.5%) in both pediatric patients (< 12 years) and adolescents, although with a higher complication rate in the group younger than 12 years of age (5.4%) and a slightly higher recurrence rate (25.5% vs 17.6%).10 Furthermore, the effectiveness of ablation varied according to the substrate treated: highest in ablation of nodal reentrant tachycardia (effectiveness 95%-99%), followed by ablation of left lateral pathways (effectiveness > 95% in all series), and focal atrial tachycardias (effectiveness 93%). In contrast, the right lateral, right septal, and left septal pathways show lower effectiveness values ranging from 80% to 90%, similar to those obtained in VT ablation (effectiveness around 75%-80%).
Cryoablation use for the treatment of various substrates has been described in several studies in pediatric patients, with immediate effectiveness rates of 83% to 98% for nodal reentrant tachycardia, which is somewhat lower than the values obtained with radiofrequency (95%-100%). These procedures are also associated with a higher rate of recurrent arrhythmias than radiofrequency ablation: between 0% and 28%, depending on the series. These data concur with those reported in a recent meta-analysis comparing radiofrequency ablation and cryoablation for the treatment of nodal reentrant tachycardia. Specifically, initial overall effectiveness was nonsignificantly lower with cryotherapy (relative risk [RR] = 1.44; 95% confidence interval [95%CI], 0.91-2.28), but cryotherapy had a higher associated recurrence rate (RR = 3.66; 95%CI, 1.84-7.28).11
In patients with CHD, ablation of accessory pathways provides slightly poorer results than those reported for patients without CHD (effectiveness 80%-85%12). The effectiveness of ablation for macroreentrant atrial tachycardia is also lower in pediatric patients with CHD than in those without (66%-97%), and recurrence rates are higher (10%-60%). The results seem to be poorer in patients with transposition of the great arteries repaired using the Senning/Mustard techniques, in those with single ventricle heart defects, patients undergoing surgical repair at advanced ages, and those treated with procedures involving nonirrigated catheters. These data are similar to those obtained in adult patients with CHD undergoing atrial arrhythmia ablation.13
The most severe immediate adverse effects are permanent atrioventricular block, an acute coronary lesion, cardiac perforation, vascular lesions, and even procedure-related death. Stochastic effects of radiation are included in the long-term adverse effects. Considering that medical therapy is avoided or discontinued in more than 60% of pediatric patients who have undergone ablation and that the majority have no other comorbidities,7 a severe complication could have particularly dramatic consequences for the quality of life of these children. Thus, it is especially important to prioritize drug therapy for the smallest patients, maximize safety measures during the ablation procedure, use electroanatomic navigation systems, and render the technique less aggressive by nonirrigated catheter use, cryoablation therapy in substrates near the conduction system, and limiting the number, duration, and energy of the applications.14
Current Situation Of Pediatric Ablation In SpainAlthough the use of ablation techniques in the pediatric age has a long history in Spain, data have been available from only a few centers up to now.15,16 The initial experience with ablation in 5 infants younger than 1 year was reported in 1997,15 and in 1998, an initial experience in a larger case series was described: 117 patients younger than 18 years, with an immediate effectiveness rate of 93% and a recurrence rate of 8%.16 The recent article by Alonso-García et al.17 published in Revista Española de Cardiología is very welcome for several reasons, but particularly because it includes a large population and describes a 12-year experience in a referral center. The findings emphasize the importance of performing these procedures in centers equipped with an arrhythmia unit with extensive experience in all substrates in CHD patients, a pediatric cardiology department with special dedication to arrhythmias, a pediatric surgery department, a pediatric anesthesia department particularly devoted to pediatric cardiology, and a stable program of collaboration between all these units. The authors should be congratulated for their excellent success and complication rates, which are in accordance with data from the literature. Without a doubt, this study supports the idea that pediatric ablation can be implemented successfully, but always in centers meeting these characteristics.
As one of the differential aspects between ablations performed in the adult and pediatric populations is the indications, it is interesting to note the small percentage of treated patients weighing less than 15kg, which account for only 7.6% of cases in the reported series. These data are in line with the current recommendations regarding these patients. Even so, it would be interesting to have information on the indications for these ablations and the drug therapy used before ablation was indicated.
One relevant factor in the article by Alonso-García et al.17 is the use of cryoablation in 35.5% of the patients. In this study and in the literature, the effectiveness and recurrence rates associated with cryoablation are higher than with radiofrequency; hence, it would have been interesting to have a description of the indications for the use of one or other energy source, as well as some details regarding the selection of the energy source in the discussion, considering that the authors report a very low atrioventricular block rate in ablations for nodal reentrant tachycardia. It would also have been of interest to have additional information on the indications for using navigation systems.
Of special note, pediatric ablations, including this series, have been recorded separately in the Spanish ablation registry for the last 2 years. Data from the 2016 registry18 show that pediatric procedures accounted for only 2.3% of the total and were performed in numerous centers. The professionals responsible for the ablation registry and the centers that carry out these procedures and record the results should be congratulated for this initiative, which raises awareness that the indications for children should be differentiated from those of adults. It also underscores the need to separately consider the success and complication rates in the 2 populations. Another fact reflected in the registry is the dispersion regarding care for pediatric patients in our country, with 369 ablation procedures carried out in 35 centers. Considering the importance of having a sufficient case volume to minimize the potentially negative effect of the learning curve in these patients,19 perhaps we should consider centralizing these procedures in referral centers. These should have fully equipped electrophysiology units and a high volume of cases, cardiology departments for pediatric patients and adults with teams experienced in CHD and equipped for radiofrequency ablation, cryoablation and electroanatomic navigation, a pediatric surgery team available 24/7, and pediatric intensive care units with experience in the management of patients with heart disease.
Conflicts Of InterestNone declared.
.