The Rubicon river marked the border between the territories of Rome and Gaul. Crossing it was, therefore, completely forbidden for armed soldiers. Crossing the river demonstrated a provocative attitude, with potentially serious consequences that could eventually spark conflict. Thus, “crossing the Rubicon” became an expression that reflected a decision to proceed onwards, with no turning back, accepting the potential conflict resulting from that decision – alea jacta est. In 2009, Dr Fernando Alfonso aptly used this expression as the title for an editorial comment on the implantation of drug-eluting stents (DES) in the left main coronary artery (LM).1
The LM was for many years a forbidden land in the world of percutaneous coronary intervention (PCI), except in extreme emergency situations or absolute inoperability. The arrival of DES marked a golden opportunity, and just a few years after becoming available, several registries were published with encouraging results that prompted the development of randomized trials in the context of LM disease.
THE SCIENTIFIC EVIDENCE: CLINICAL TRIALSThe SYNTAX trial began in 2005 and included 705 patients in the group with LM disease randomized to either PCI with first-generation paclitaxel DES or coronary artery bypass graft (CABG). The primary outcome at 5 years showed no differences between groups, but in the subgroup with complex cardiac anatomy, SYNTAX score ≥ 33, the cumulative event rate was clearly lower in the surgical arm.2 The 10-year outcomes of this study have since been published, and no differences were observed in total mortality between PCI and CABG.3
In the PRECOMBAT trial, with 600 patients and first-generation sirolimus DES in the PCI arm, the 5-year results showed no differences in the primary outcome, but did show differences in the rate of revascularization of the treated lesion, which was lower with CABG.4 These findings were maintained at 10-year follow-up.5
First-generation DES were associated with a certain incidence of late thrombosis and were progressively relegated by second-generation DES, which had a better efficacy and safety profile. Two large trials in the field of LM were designed: NOBLE and EXCEL.6,7
The NOBLE trial included 1201 patients randomized to CABG or PCI with biolimus DES. At the 5-year follow-up, the total mortality and cardiac mortality were similar, but the primary outcome favored CABG, fundamentally due to a significantly lower incidence of revascularization.6
The EXCEL trial included 1905 patients with SYNTAX score ≤ 32 who were randomized to PCI with everolimus DES or CABG. PCI was shown to be not inferior to CABG for the primary outcome of all-cause mortality, stroke, or acute myocardial infarction (AMI) at 3 years, even after adding revascularization of the treated vessel.7
The contrast between these 2 studies can be explained by the differences in the definition of the primary outcome, the exclusion of periprocedural AMI (pAMI) in NOBLE, the different follow-up times, the higher incidence of thrombosis observed in NOBLE (3% vs 0.7%), and the higher incidence of stroke in the PCI arm of NOBLE, which was seen from the second year and attributed to chance, due to both its very late presentation and the opposite trend shown in the meta-analysis.8
Following the results of these trials, the 2018 European clinical practice guidelines for myocardial revascularization supported PCI of the LM, with a class I recommendation and level of evidence A if SYNTAX score was ≤ 22, and class IIa recommendation with level of evidence A if SYNTAX score was 23-32.9
When it seemed that this “story” had come to an end, in 2019, the 5-year results of the EXCEL trial were published.10 Although the primary outcome was similar, 22% with PCI and 19.2% with CABG (P=.13), all-cause mortality was higher in the PCI group (13% vs 9.9%; P=.04), with comparable incidences of cardiovascular death and AMI. The curves for the primary outcome crossed after 3 years. There were fewer strokes after PCI, but revascularization due to ischemia was significantly more frequent.
This difference in total mortality, something which was not observed in other trials or meta-analyses,8 revived the controversy, made even more intense with the definition of pAMI used in the trial. The statements of a British surgeon, Dr David Taggart,11 on a news program generated much ado in the media. For the first time, disagreement among the scientific societies of cardiac surgeons and cardiologists made it to the mainstream press, generating confusion and some alarm in professional sectors and, most worrying of all, among patients.
ALL-CAUSE MORTALITY AND CARDIOVASCULAR MORTALITYIn the trials performed, with 5- and 10-year follow-up available, both total mortality and cardiovascular mortality are perfectly comparable for PCI and CABG.8
Total mortality at 10 years was similar in the SYNTAX and PRECOMBAT trials, and also in the NOBLE trial at 5 years, the only difference being in the EXCEL trial at 5 years, with an absolute difference of 3.1%.3,5,6,10 This outcome did not form part of the study design; in fact, it was not specified in the hypothesis test; furthermore, cardiovascular mortality was almost identical, and the differences were explained by late deaths caused by infection and cancer.7,10
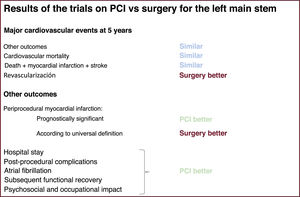
To improve the accuracy of the results for low-frequency outcomes (eg, death, AMI, stroke), it is necessary to analyze all the data from high-quality studies. At the time of writing this editorial, the most recent meta-analysis is that published by Ahmad et al.8 in the European Heart Journal. This included 5 trials with a total of 4612 patients and a mean follow-up of 5.6 years. There were no significant differences between PCI and CABG in terms of risk of death from all causes (risk ratio =1.03; 95% confidence interval [95%CI], 0.81-1.32; P=.77) or cardiac death. There were also no significant differences in the risk of stroke or AMI. PCI was associated with a higher risk of unplanned revascularization (figure 1).
Comparison between left main stem revascularization techniques for different outcomes according to the trials and their meta-analyses.2–8,10,12 PCI, percutaneous coronary intervention.
The definition of pAMI in the EXCEL protocol was agreed by consensus, including the surgical committee, who considered it a priority to eliminate the confirmation bias for each revascularization technique. The definition is similar to that used in the SYNTAX trial, which has never been questioned and predates and is different to that of the Society for Cardiovascular Angiography and Intervention (SCAI).
It was unanimously agreed to use a definition that had demonstrated prognostic significance, eliminating confirmation bias for PCI/CABG, and in which the cutoffs for raised biomarkers would be identical for both techniques, naturally. The definition was evidence-based, rather than being arbitrary, as the cutoff values for the MB isoenzyme of creatine kinase (CK-MB) used represent degrees of necrosis that have been independently associated with risk of death after PCI and CABG in the EXCEL study itself.13 Furthermore, the same additional criteria were used based on symptoms, electrocardiography, angiography, and noninvasive imaging techniques.
The decision not to use the third universal definition of AMI after PCI (type 4 a) and after CABG (type 5)14 was based on several factors: a) this definition uses arbitrarily different biomarker cutoffs, and requires twice the enzyme level to qualify as an infarction after CABG than after PCI, and b) there is disparity in the additional criteria: symptoms are a criterion after PCI but not after CABG, the ischemic changes on ECG required after PCI can be changes in ST, T, or Q, compared with the presence of Q waves after CABG, angiography is performed in 100% of patients after PCI but rarely after CABG, and even then the angiographic criteria are stricter after CABG than PCI.
These points have been corroborated in a recent analysis of the EXCEL trial carried out to evaluate the implications of the possible definitions of pAMI after coronary revascularization.12 Periprocedural AMI according to the protocol definition occurred in 3.6% after PCI and in 6.1% after CABG (P=.015). The corresponding rates of pAMI according to the universal definition were 4.0% and 2.2%, respectively (P=.025). Periprocedural AMI according to the protocol definition was associated with a persistent risk of cardiovascular mortality after PCI and CABG (P of interaction=.86); in contrast, pAMI according to the universal definition had a strong association with cardiovascular mortality after CABG (adjusted RR=11.9; 95%CI, 4.8-29.4) but not after PCI (adjusted RR=1.1; 95%CI, 0.3-3.6; P of interaction=.004). Only large increases in biomarkers (CK-MB ≥ 10 × the reference upper limit and troponin ≥ 70 × the reference upper limit) were associated with mortality.
Therefore, although the rates of pAMI after PCI and CABG varied greatly with the different definitions, pAMI as defined in the EXCEL protocol was associated with a similar prognostic risk after PCI and CABG, whereas pAMI as defined by the universal definition had a strong association with mortality after CABG, but not after PCI.
CONSIDERATIONS REGARDING TRIALS AND REPRODUCTION OF RESULTS IN CLINICAL PRACTICEOne very important aspect is that concerning operative mortality. Surgical mortality is more concentrated in the acute phase than PCI mortality, so its trial outcomes are less reproducible in clinical practice than those of PCI. Also, the variability in surgical mortality is higher than the existing PCI mortality, as the surgical act is more complex, more multifactorial, and therefore, more susceptible to complications.
The volume of CABGs performed in each center and by each operator has decreased substantially in the past 2 decades, due to the growth of PCI and the opening of surgical services in multiple centers. In contrast, the volume of PCIs performed by each operator is high, and experience in PCI of the LM can draw upon the experience with PCI at other locations, such as bifurcations of the coronary tree outside the main stem, which can be technically even more demanding.15
The surgical technique used in trials is also less reproducible in practice than that of PCI. The CABG performed in trials differs from real-world practice, with the trials having much higher rates of complete arterial revascularization. The use of saphenous grafts, which is very high in trials, is even higher in clinical practice. This is important, as the patency of these grafts is limited over time, with only half of them remaining functional at 10 years and, of these, half have lesions.
In all the above trials, there has been a reference arm for patients who were not randomized because they were considered unsuitable for either technique. In addition, as is well known, the profile of patients included in trials of PCI vs CABG is that of lower surgical risk than in clinical practice, as advanced age and comorbidities are dissuasive factors for eligibility.16
Finally, while the CABG technique has undergone few significant changes in recent decades, PCI has been and is undergoing constant changes. This is demonstrated by the ever improving design of DES, the use of optimization protocols for PCI of the LM with intravascular imaging,17 new plaque modification techniques, the original bifurcation stenting strategies, percutaneous circulatory assist devices, and a more optimal pharmacological treatment, in terms of antiplatelet therapy and control of cardiovascular risk factors. Each of these separate factors has been shown to have a positive effect on prognosis after PCI.
BEYOND PRIMARY OUTCOMES AND MAJOR CARDIAC EVENTSIn the analysis of these trials, not enough prominence was given to other aspects that, assuming equivalence of harder clinical outcomes such as death, AMI, or stroke, are very relevant (figure 1).
CABG requires a longer hospital stay (in postoperative units and on the ward). The surgical intervention has a higher rate of complications, such as bleeding and consequent need for transfusion of blood products, infections and their treatment with antibiotics, renal damage, or atrial fibrillation, and therefore has a higher use of resources.7 The psychosocial and occupational impact is also higher, and functional recovery takes longer after CABG, which in older patients can be very important.
However, PCI requires more repeat revascularization procedures during follow-up. With CABG, although these procedures are done less frequently, they are necessary for a longer time after the CABG, and are likely to be more complex because they involve treatment of degenerated saphenous grafts or very distal lesions in native vascular beds.
Therefore, when comparing these 2 techniques, in addition to the hard clinical outcomes, other effects and consequences of each technique must be taken into account. Patients want to live longer, but also live well.
CONCLUSIONThe scientific evidence demonstrates that revascularization of the LM is no longer exclusively done by a surgical approach, and the percutaneous approach is a valid alternative and even more appropriate for a large proportion of patients.
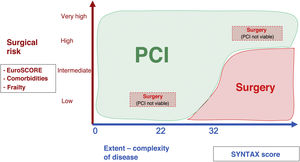
The surgical risk, extent of coronary disease and patient preference will also be key in determining which technique the cardiology team opts to perform (figure 2). The clinical cardiologist knows the patient best and their position is essential in decision making. Clearly the demographic changes of the patient population will make PCI increasingly suitable. Both percutaneous and surgical revascularization must conform to the highest standards of quality, with knowledge and follow-up of local outcomes.
This Rubicon was crossed years ago and there is no way back now. What we as cardiologists and surgeons must do is leave aside the controversies, so often stimulated more by “professional partisaniship” than by serious scientific arguments, and together move forward toward comprehensive excellence in the treatment of this important disease.
All for the good of the patients.
CONFLICTS OF INTERESTJ.M. de la Torre Hernández has received unconditional research grants from Abbott Medical, Amgen, and IHT, and honorarium for consultation and educational sessions from Abbott Medical, Boston Scientific, Medtronic, Biotronik, Astra Zeneca, Ferrer, Terumo, Daiichi-Sankyo, and BMS.