Type 2 diabetes mellitus (DM2) is a common comorbidity in patients with heart failure (HF) with preserved ejection fraction (HFpEF). Previous studies have shown that diabetic women are at higher risk of developing HF than men. However, the long-term prognosis of diabetic HFpEF patients by sex has not been extensively explored. In this study, we aimed to evaluate the differential impact of DM2 on all-cause mortality in men vs women with HFpEF after admission for acute HF.
MethodsWe prospectively included 1019 consecutive HFpEF patients discharged after admission for acute HF in a single tertiary referral hospital. Multivariate Cox regression analysis was used to evaluate the interaction between sex and DM2 regarding the risk of long-term all-cause mortality. Risk estimates were calculated as hazard ratios (HR).
ResultsThe mean age of the cohort was 75.6±9.5 years and 609 (59.8%) were women. The proportion of DM2 was similar between sexes (45.1% vs 49.1, P=.211). At a median (interquartile range) follow-up of 3.6 (1-4-6.8) years, 646 (63.4%) patients died. After adjustment for risk factors, comorbidities, biomarkers, echo parameters and treatment at discharge, multivariate analysis showed a differential prognostic effect of DM2 (P value for interaction=.007). DM2 was associated with a higher risk of all-cause mortality in women (HR, 1.77; 95%CI, 1.41-2.21; P <.001) but not in men (HR, 1.23; 95%CI, 0.94-1.61; P=.127).
ConclusionsAfter an episode of acute HF in HFpEF patients, DM2 confers a higher risk of mortality in women. Further studies evaluating the impact of DM2 in women with HFpEF are warranted.
Keywords
Heart failure (HF) with preserved left ventricular ejection fraction (HFpEF) constitutes the most prevalent form of HF in women and in the elderly.1 Type 2 diabetes mellitus (DM2) is a common comorbidity in these patients and is associated with worse prognosis.2–4
The effect of DM2 on cardiovascular complications seems to have a sex-specific interaction. Female sex confers special protection against cardiovascular disease, but this protection is lost in the presence of DM2.5 Diabetes is also associated with a significant increase in HF incidence in men and women, although diabetic women have a greater relative risk of developing HF than men.6 Nevertheless, there is little research exploring differences in prognosis between men and women in DM2 with established HFpEF. Although there are more women with HFpEF than men, women are still underrepresented in clinical trials and many studies are underpowered to explore sex-specific differences. This situation is expected to be corrected by the inclusion of sex as an important independent biological variable by the US National Institute of Health, but the scientific information available at the time being is still limited.7 Observational registries play an important role in providing scientific evidence in patients who are not well represented in randomized clinical trials. We therefore aimed to evaluate the differential impact of DM2 on the risk of all-cause and cause-specific mortality in men vs women with HFpEF following hospitalization for acute heart failure (AHF).
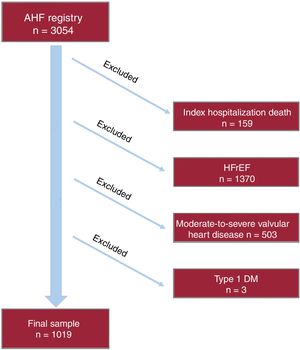
METHODSStudy design and patientsThis prospective observational cohort study included 3054 consecutive patients admitted with AHF in the cardiology department of a tertiary referral hospital from January 1, 2004 to August 1, 2016. AHF was defined according to clinical practice guidelines.8–10 Patients with new-onset or acutely decompensated HF were included in the registry. Patients who died during the index hospitalization were excluded from the final analysis. In addition, patients with left ventricular ejection fraction <50%, significant (moderate to severe primary valvular disease), and those with type 1 diabetes were also excluded from the present analysis, as shown in the flow chart (figure 1). The final study sample included 1019 HFpEF patients. During the index hospitalization, data on demographics, medical history, vital signs, 12-lead electrocardiogram, laboratory and echocardiographic parameters, and drug use were routinely recorded using pre-established registry questionnaires. Left ventricular ejection fraction was assessed by 2-dimensional echocardiography and calculated by the biplane Simpson method (96±24hours after admission). Two commercially available systems were used throughout the study: Agilent Sonos 5500 and ie33 (Philips, Massachusetts, United States). HFpEF during admission was defined as a left ventricular ejection fraction of 50% or more, echocardiographic structural or functional abnormalities (left atrial enlargement or left ventricular hypertrophy), and elevated natriuretic peptides based on current clinical practice guidelines.8–10 Treatment at discharge was also recorded.
Type 2 diabetes mellitus status by sexDiagnosis of DM2 was determined by reviewing patients’ medical records during the index hospitalization. DM2 was evaluated as a dichotomous variable, and patients were classified according to sex in 4 groups: a) non-DM2 men, b) DM2 men, c) non-DM2 women, and d) DM2 women.
Ethics concernsThe study conformed to the principles outlined in the Declaration of Helsinki and was approved by the local institutional ethics committee (Hospital Clínico Universitario de Valencia). All patients gave informed consent.
Endpoint and follow-upThe primary endpoint was the incidence of all-cause mortality during follow-up. Secondary endpoints were cardiovascular mortality. Cardiovascular mortality included sudden death, progressive HF death, deaths attributable to other cardiovascular causes (such as acute coronary syndrome and stroke), and unknown cause of death. The personnel in charge of endpoint adjudications during follow-up were blinded to DM2 status.
Statistical analysisContinuous variables are presented as mean ± standard deviation or median (interquartile range), as appropriate. Categorical variables are expressed as percentages. Baseline continuous variables were compared by DM2 status with the Student t test or rank-sum test as appropriate; discrete variables were compared with the chi-square test. The interaction between sex and DM2 status during follow-up was evaluated by Cox regression analysis for the primary endpoint and Cox adapted for competing risk for cardiovascular mortality.11 Risk estimates are expressed as hazard ratios (HRs) with 95% confidence intervals (95%CIs). All variables listed in table 1 were evaluated as potential confounders. The covariates included in the multivariate model were selected based on previous knowledge/biological plausibility independently of the P value. The linearity assumption for all continuous variables was simultaneously tested and the variable transformed, if appropriate, with fractional polynomials. Then, a reduced and parsimonious model was derived by using backward stepdown selection. Proportionality assumption for the hazard function over time was tested by means of the Schoenfeld residuals. Discriminative abilities of the multivariate models were evaluated with Harrell's c-statistics. The final multivariate model for the primary endpoint included the following covariates: age, body mass index, prior admission for AHF, ischemic heart disease, Charlson index, heart rate, atrial fibrillation, estimated glomerular filtration rate, hemoglobin, sodium, N-terminal pro-B-type natriuretic peptide, left atrial diameter, and HF treatment (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, aldosterone antagonists or beta-blockers). Harrell's c-statistic of the model was 0.713. For cardiovascular mortality, the final model included the following covariates: age, body mass index, prior admission for AHF, estimated glomerular filtration rate, sodium, N-terminal pro-B-type natriuretic peptide, and HF treatment at discharge (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, aldosterone antagonists or beta-blockers). Harrell's c-statistic of the model was 0.701.
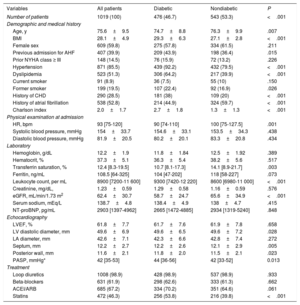
Baseline characteristics by sex in HFpEF patients
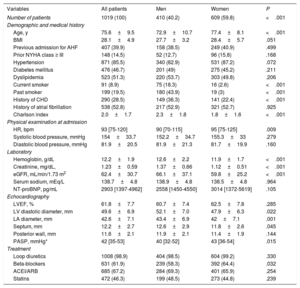
| Variables | All patients | Men | Women | P |
|---|---|---|---|---|
| Number of patients | 1019 (100) | 410 (40.2) | 609 (59.8) | <.001 |
| Demographic and medical history | ||||
| Age, y | 75.6±9.5 | 72.9±10.7 | 77.4±8.1 | <.001 |
| BMI | 28.1±4.9 | 27.7±3.2 | 28.4±5.7 | .051 |
| Previous admission for AHF | 407 (39.9) | 158 (38.5) | 249 (40.9) | .499 |
| Prior NYHA class ≥ III | 148 (14.5) | 52 (12.7) | 96 (15.8) | .168 |
| Hypertension | 871 (85.5) | 340 (82.9) | 531 (87.2) | .072 |
| Diabetes mellitus | 476 (46.7) | 201 (49) | 275 (45.2) | .211 |
| Dyslipidemia | 523 (51.3) | 220 (53.7) | 303 (49.8) | .206 |
| Current smoker | 91 (8.9) | 75 (18.3) | 16 (2.6) | <.001 |
| Past smoker | 199 (19.5) | 180 (43.9) | 19 (3) | <.001 |
| History of CHD | 290 (28.5) | 149 (36.3) | 141 (22.4) | <.001 |
| History of atrial fibrillation | 538 (52.8) | 217 (52.9) | 321 (52.7) | .925 |
| Charlson index | 2.0±1.7 | 2.3±1.8 | 1.8±1.6 | <.001 |
| Physical examination at admission | ||||
| HR, bpm | 93 [75-120] | 90 [70-115] | 95 [75-125] | .009 |
| Systolic blood pressure, mmHg | 154±33.7 | 152.2±34.7 | 155.3±33 | .279 |
| Diastolic blood pressure, mmHg | 81.9±20.5 | 81.9±21.3 | 81.7±19.9 | .160 |
| Laboratory | ||||
| Hemoglobin, g/dL | 12.2±1.9 | 12.6±2.2 | 11.9±1.7 | <.001 |
| Creatinine, mg/dL, | 1.23±0.59 | 1.37±0.66 | 1.12±0.51 | <.001 |
| eGFR, mL/min/1.73 m2 | 62.4±30.7 | 66.1±37.1 | 59.8±25.2 | <.001 |
| Serum sodium, mEq/L | 138.7±4.8 | 138.9±4.8 | 138.5±4.8 | .964 |
| NT-proBNP, pg/mL | 2903 [1397-4962] | 2558 [1450-4550] | 3014 [1372-5619] | .105 |
| Echocardiography | ||||
| LVEF, % | 61.8±7.7 | 60.7±7.4 | 62.5±7.8 | .285 |
| LV diastolic diameter, mm | 49.6±6.9 | 52.1±7.0 | 47.9±6.3 | .022 |
| LA diameter, mm | 42.6±7.1 | 43.4±6.9 | 42±7.1 | .001 |
| Septum, mm | 12.2±2.7 | 12.6±2.9 | 11.8±2.6 | .045 |
| Posterior wall, mm | 11.6±2.1 | 11.9±2.1 | 11.4±1.9 | .144 |
| PASP, mmHg* | 42 [35-53] | 40 [32-52] | 43 [36-54] | .015 |
| Treatment | ||||
| Loop diuretics | 1008 (98.9) | 404 (98.5) | 604 (99.2) | .330 |
| Beta-blockers | 631 (61.9) | 239 (58.3) | 392 (64.4) | .032 |
| ACEI/ARB | 685 (67.2) | 284 (69.3) | 401 (65.9) | .254 |
| Statins | 472 (46.3) | 199 (48.5) | 273 (44.8) | .239 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AHF, acute heart failure; BMI, body mass index; CHD, coronary heart disease; eGFR: estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
Data are expressed as mean±standard deviation, No. (%), or median [interquartile range].
A sensitivity analysis was performed only in DM2 patients and included multivariate adjustment for age, body mass index, Charlson index, atrial fibrillation, heart rate, glomerular filtration rate, sodium, N-terminal pro-B-type natriuretic peptide, glycemic control (glycosylated hemoglobin [HbA1c]), and antidiabetic treatment at discharge (insulin, metformin, sulphonylureas, and dipeptidyl peptidase-4 inhibitors).
Two tailed P values less than .05 were statistically significant in all analyses. All statistical analyses were performed using STATA 14.1 (StataCorp. 2014. Stata Statistical Software: Release 14.1. College Station, TX: StataCorp LP).
RESULTSBaseline characteristicsOf the 1019 patients with HFpEF included in this study, 476 (46.7%) had DM2, with a similar prevalence between sexes (45.1% vs 49.1%; P=.211). The mean age of the cohort was 75.6±9.5 years, 609 (59.8%) were women and 407 (39.9%) had been previously admitted for AHF. The median (interquartile range) N-terminal pro-B-type natriuretic peptide level was 2903 (1397-4962) pg/mL.
Table 1 and table 2 summarize the baseline characteristics of all patients stratified by sex and DM2, respectively. Differences between groups were found in clinical, biochemical, and echocardiographic parameters. Overall, women were older, had a higher heart rate, a lower mean estimated glomerular filtration rate, and higher pulmonary artery pressures. Likewise, they showed a trend to higher body mass index. In contrast, women had fewer comorbidities and were more frequently treated with beta-blockers and less frequently with angiotensin-converting enzyme inhibitors at discharge.
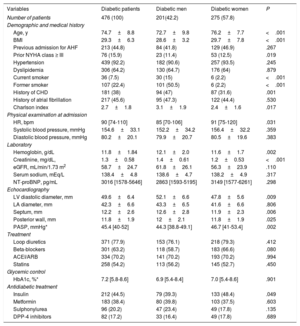
Baseline characteristics by diabetes in HFpEF patients
| Variables | All patients | Diabetic | Nondiabetic | P |
|---|---|---|---|---|
| Number of patients | 1019 (100) | 476 (46.7) | 543 (53.3) | <.001 |
| Demographic and medical history | ||||
| Age, y | 75.6±9.5 | 74.7±8.8 | 76.3±9.9 | .007 |
| BMI | 28.1±4.9 | 29.3±6.3 | 27.1±2.8 | <.001 |
| Female sex | 609 (59.8) | 275 (57.8) | 334 (61.5) | .211 |
| Previous admission for AHF | 407 (39.9) | 209 (43.9) | 198 (36.4) | .015 |
| Prior NYHA class ≥ III | 148 (14.5) | 76 (15.9) | 72 (13.2) | .226 |
| Hypertension | 871 (85.5) | 439 (92.2) | 432 (79.5) | <.001 |
| Dyslipidemia | 523 (51.3) | 306 (64.2) | 217 (39.9) | <.001 |
| Current smoker | 91 (8.9) | 36 (7.5) | 55 (10) | .150 |
| Former smoker | 199 (19.5) | 107 (22.4) | 92 (16.9) | .026 |
| History of CHD | 290 (28.5) | 181 (38) | 109 (20) | <.001 |
| History of atrial fibrillation | 538 (52.8) | 214 (44.9) | 324 (59.7) | <.001 |
| Charlson index | 2.0±1.7 | 2.7±1.8 | 1.3±1.3 | <.001 |
| Physical examination at admission | ||||
| HR, bpm | 93 [75-120] | 90 [74-110] | 100 [75-127.5] | .001 |
| Systolic blood pressure, mmHg | 154±33.7 | 154.6±33.1 | 153.5±34.3 | .438 |
| Diastolic blood pressure, mmHg | 81.9±20.5 | 80.2±20.1 | 83.3±20.8 | .434 |
| Laboratory | ||||
| Hemoglobin, g/dL | 12.2±1.9 | 11.8±1.84 | 12.5±1.92 | .389 |
| Hematocrit, % | 37.3±5.1 | 36.3±5.4 | 38.2±5.6 | .517 |
| Transferrin saturation, % | 12.4 [8.3-19.5] | 10.7 [8.1-17.3] | 14.1 [8.9-21.7] | .003 |
| Ferritin, ng/mL | 108.5 [64-325] | 104 [47-202] | 118 [58-227] | .073 |
| Leukocyte count, per mL | 8900 [7200-11 600] | 9300 [7420-12 220] | 8600 [6980-11 000] | <.001 |
| Creatinine, mg/dL, | 1.23±0.59 | 1.29±0.58 | 1.16±0.59 | .576 |
| eGFR, mL/min/1.73 m2 | 62.4±30.7 | 58.7±24.7 | 65.6±34.9 | <.001 |
| Serum sodium, mEq/L | 138.7±4.8 | 138.4±4.9 | 138±4.7 | .415 |
| NT-proBNP, pg/mL | 2903 [1397-4962] | 2665 [1472-4885] | 2934 [1319-5240] | .848 |
| Echocardiography | ||||
| LVEF, % | 61.8±7.7 | 61.7±7.6 | 61.9±7.8 | .658 |
| LV diastolic diameter, mm | 49.6±6.9 | 49.6±6.5 | 49.6±7.2 | .028 |
| LA diameter, mm | 42.6±7.1 | 42.3±6.6 | 42.8±7.4 | .272 |
| Septum, mm | 12.2±2.7 | 12.2±2.6 | 12.1±2.9 | .005 |
| Posterior wall, mm | 11.6±2.1 | 11.8±2.0 | 11.5±2.1 | .023 |
| PASP, mmHg* | 42 [35-53] | 44 [36-56] | 42 [33-52] | 0.013 |
| Treatment | ||||
| Loop diuretics | 1008 (98.9) | 428 (98.9) | 537 (98.9) | .933 |
| Beta-blockers | 631 (61.9) | 298 (62.6) | 333 (61.3) | .662 |
| ACEI/ARB | 685 (67.2) | 334 (70.2) | 351 (64.6) | .061 |
| Statins | 472 (46.3) | 256 (53.8) | 216 (39.8) | <.001 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AHF, acute heart failure; BMI, body mass index; CHD:, coronary heart disease; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrium; LV, left ventricle; NT-proBNP, N-terminal-pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
Data are expressed as mean±standard deviation, No. (%), or median [interquartile range].
Patients with DM2 had more cardiovascular risk factors, more frequent prior HF hospitalizations, a higher burden of comorbidities and greater HF severity. Diabetic patients received a higher proportion of ACEI/ARB and statins at discharge. Among patients with DM2, women were older, had a higher body mass index, and worse baseline functional class (table 3). Regarding echocardiographic parameters, women had a lower left ventricular diameter, lower left ventricular wall thickness, and higher pulmonary artery pressures.
Baseline characteristics by sex in patients with type 2 diabetes mellitus and HFpEF
| Variables | Diabetic patients | Diabetic men | Diabetic women | P |
|---|---|---|---|---|
| Number of patients | 476 (100) | 201(42.2) | 275 (57.8) | |
| Demographic and medical history | ||||
| Age, y | 74.7±8.8 | 72.7±9.8 | 76.2±7.7 | <.001 |
| BMI | 29.3±6.3 | 28.6±3.2 | 29.7±7.8 | <.001 |
| Previous admission for AHF | 213 (44.8) | 84 (41.8) | 129 (46.9) | .267 |
| Prior NYHA class ≥ III | 76 (15.9) | 23 (11.4) | 53 (12.5) | .019 |
| Hypertension | 439 (92.2) | 182 (90.6) | 257 (93.5) | .245 |
| Dyslipidemia | 306 (64.2) | 130 (64.7) | 176 (64) | .879 |
| Current smoker | 36 (7.5) | 30 (15) | 6 (2.2) | <.001 |
| Former smoker | 107 (22.4) | 101 (50.5) | 6 (2.2) | <.001 |
| History of CHD | 181 (38) | 94 (47) | 87 (31.6) | .001 |
| History of atrial fibrillation | 217 (45.6) | 95 (47.3) | 122 (44.4) | .530 |
| Charlson index | 2.7±1.8 | 3.1±1.9 | 2.4±1.6 | .017 |
| Physical examination at admission | ||||
| HR, bpm | 90 [74-110] | 85 [70-106] | 91 [75-120] | .031 |
| Systolic blood pressure, mmHg | 154.6±33.1 | 152.2±34.2 | 156.4±32.2 | .359 |
| Diastolic blood pressure, mmHg | 80.2±20.1 | 79.9±20.7 | 80.5±19.6 | .383 |
| Laboratory | ||||
| Hemoglobin, g/dL | 11.8±1.84 | 12.1±2.0 | 11.6±1.7 | .002 |
| Creatinine, mg/dL, | 1.3±0.58 | 1.4±0.61 | 1.2±0.53 | <.001 |
| eGFR, mL/min/1.73 m2 | 58.7±24.7 | 61.8±26.1 | 56.3±23.9 | .110 |
| Serum sodium, mEq/L | 138.4±4.8 | 138.6±4.7 | 138.2±4.9 | .317 |
| NT-proBNP, pg/mL | 3016 [1578-5646] | 2863 [1593-5195] | 3149 [1577-6261] | .298 |
| Echocardiography | ||||
| LV diastolic diameter, mm | 49.6±6.4 | 52.1±6.6 | 47.8±5.6 | .009 |
| LA diameter, mm | 42.3±6.6 | 43.3±6.5 | 41.6±6.6 | .806 |
| Septum, mm | 12.2±2.6 | 12.6±2.8 | 11.9±2.3 | .006 |
| Posterior wall, mm | 11.8±1.9 | 12±2.1 | 11.8±1.9 | .025 |
| PASP, mmHg* | 45.4 [40-52] | 44.3 [38.8-49.1] | 46.7 [41-53.4] | .002 |
| Treatment | ||||
| Loop diuretics | 371 (77.9) | 153 (76.1) | 218 (79.3) | .412 |
| Beta-blockers | 301 (63.2) | 118 (58.7) | 183 (66.6) | .080 |
| ACEI/ARB | 334 (70.2) | 141 (70.2) | 193 (70.2) | .994 |
| Statins | 258 (54.2) | 113 (56.2) | 145 (52.7) | .450 |
| Glycemic control | ||||
| HbA1c, %* | 7.2 [5.8-8.6] | 6.9 [5.4-8.4] | 7.0 [5.4-8.6] | .901 |
| Antidiabetic treatment | ||||
| Insulin | 212 (44.5) | 79 (39.3) | 133 (48.4) | .049 |
| Metformin | 183 (38.4) | 80 (39.8) | 103 (37.5) | .603 |
| Sulphonylurea | 96 (20.2) | 47 (23.4) | 49 (17.8) | .135 |
| DPP-4 inhibitors | 82 (17.2) | 33 (16.4) | 49 (17.8) | .689 |
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; AHF, acute heart failure; BMI, body mass index; CHD, coronary heart disease; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal-pro brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
Data are expressed as mean±standard deviation, No. (%), or median [interquartile range].
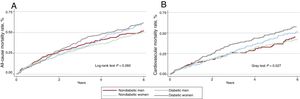
At a median follow-up of 3.6 (1-4-6.8) years, 646 (63.4%) patients had died. Mortality rates were higher in patients with DM2 (1.86 vs 1.59 per 10 person/y; P=.037). However, mortality rates were similar in male and female patients (1.70 vs 1.72 per 10 person/y; P=.888). Across DM2 and sex groups, Kaplan-Meier curves showed a statistical trend to a higher risk of mortality in diabetic women (figure 2A). These differences were found after the first year of follow-up and were highest at 3 years after discharge.
A: Kaplan-Meier of all-cause mortality curves for heart failure with preserved ejection fraction by sex and diabetes mellitus status. B: cumulative incidence functions of cardiovascular mortality for heart failure with preserved ejection fraction according to sex and diabetes mellitus status.
On multivariate analysis, after a thorough adjustment for potential confounders (including established risk factors, comorbidities, biomarkers, echo parameters, and treatments at discharge), the differential prognostic effect of DM2 across sex was significant (P value for interaction=.007). Thus, DM2 was associated with a higher risk of all-cause mortality in women (HR, 1.77; 95%CI, 1.41-2.21; P <.001) but not in men (HR, 1.23; 95%CI, 0.94-1.61; P=.127).
Cardiovascular mortalityDuring follow-up, 434 cardiovascular deaths were registered (67.2% of deaths). Across DM2 and sex groups, women with DM2 showed the highest cumulative incidence of cardiovascular mortality (figure 2B). After multivariate adjustment, including noncardiovascular mortality as a competing event and established prognosticators, women with DM2 showed an increased risk of cardiovascular mortality (HR, 1.74; 95%CI, 1.35-2.25; P <.001). However, DM2 did not confer an excess of risk in male patients (HR=1.27; 95%CI, 0.92-1.76; P=.150).
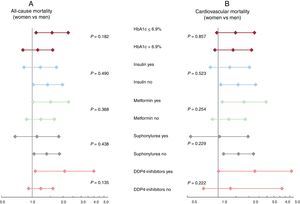
Excess mortality in women with type 2 diabetes mellitus: impact of type 2 diabetes mellitus control and treatmentAmong 476 patients with DM2, we registered 312 and 215 all-cause and cardiovascular deaths, respectively. The median HbA1c value did not differ between women and men (7% [1.6] vs 6.9% [1.5]; P=.365). Women were more frequently treated with insulin, without differences in oral antidiabetic treatments (table 3). After multivariate adjustment that included established prognostic factors and potential confounders (antidiabetic treatment and the HbA1c values), DM2 was associated with a higher risk of mortality in women, but not in men. The increase in risk was observed for all-cause mortality (HR, 1.31; 95%CI, 1.02-1.69; P=.036) and for cardiovascular mortality (HR, 1.44; 95%CI, 1.06-1.94; P=.019). Subgroup analysis revealed that the effect of sex on the risks of all-cause and cardiovascular mortality were homogenous across glycemic control and antidiabetic treatment (figure 3A, B).
DISCUSSIONThe most significant novel finding of this study is that DM2 was associated with a higher risk of all-cause and cardiovascular mortality in women with HFpEF but not in men. This effect was independent of other relevant risk factors such as age, body mass index, comorbidities, surrogates of disease severity, and HF medical treatment. In addition, excess risk in women was also found after adjustment for glycemic control and antidiabetic treatment during the index event.
Most of the existing evidence on the effect of DM2 and HF comes from patients with HF with reduced ejection fraction (HFrEF). The CHARM investigators demonstrated that DM2 was an independent predictor of morbidity and mortality in HFrEF and HFpEF, showing a greater adverse effect among those with HFpEF.12 The mortality risk conferred by DM2 was similar in HFrEF and HFpEF, but only the latter group had a greater risk of HF hospitalization. In a prospective Asian registry of patients with HFrEF, DM was associated in both sexes with higher all-cause mortality and hospitalization, and this effect was stronger in women.13
In the setting of HFpEF, prior studies have consistently shown that DM2 identifies a subset of patients with worse functional status and at higher risk of adverse clinical events.14 Nonetheless, prior studies made no distinction between women and men. Indeed, to the best of our knowledge, this is the first study postulating a more aggressive behavior of DM2 in women with HFpEF. In agreement with the present findings, and despite the differences in characteristics of the sample, a substudy of the EMPA-REG OUTCOME trial also showed that women with DM2 were hospitalized for HF more frequently than men.15
The reasons endorsing this differential effect across the sexes remain largely speculative. First, we may argue, as has been shown in other cardiovascular scenarios, that women receive less intense glycemic control and treatment of DM2 and HF compared with men.16–18 However, we believe this is not a strong argument to explain the present findings because there is no well-established treatment in HFpEF and, except for a higher prescription of beta-blockers in women, there were no differences in other HF medications at discharge. Likewise, and regarding diabetic control and treatment, similar values of HbA1c and antidiabetic treatments were found in both sexes, except for higher prescription of insulin in women. In addition, the excess of risk in women remained unaltered after adjusting for treatments and glycemic control at discharge. However, the influence of monitorization, therapeutic changes and glycemic control along the follow-up should be evaluated in further studies. Second, we may also speculate that DM2 and HF are diagnosed at later stages in women. Women frequently have confounding factors such as obesity and lower natriuretic peptides, which may delay correct diagnosis and treatment.19,20 Last, we may focus on cardiac reasons. Women with DM2 had a more severe HFpEF phenotype, with greater left ventricular diastolic dysfunction or right ventricular HF.21 Indeed, women with DM2 usually had higher systolic pulmonary artery pressures and more pronounced left ventricular hypertrophy.22,23 The biological reasons for these sex-related cardiac differences are not clear. However, sex-specific progression of diabetic cardiomyopathy has been demonstrated in studies measuring functional and structural changes in diabetic patients without structural heart disease and in cardiac patients.21,24 Basic studies with mice have confirmed these structural and functional changes and have added data at the molecular level showing that the onset of cardiac dysfunction is faster and more severe in female mice than in male mice.25 The mechanisms explaining this behavior are not fully understood, but some hypotheses have been proposed. According to one of these hypotheses, hyperglycemia may have a greater impact on left ventricular structure in women.23 Early down-regulation of the prosurvival protein Pim-1 has been proposed as a molecular cornerstone of the sex-specific progression of diabetic cardiomyopathy, indeed restoration of Pim-1 levels resulted in the reversion of molecular and structural changes, and increased the survival of female diabetic cardiomyocytes.25 This sex-differential response to DM2 has been also linked to estrogen activity.26
LimitationsOur study has several limitations. First, this is a single-center observational study with many potential confounders. Although multivariate adjustment was performed, the effects of residual confounding cannot be fully ruled out. However, the observational nature of our study provides real-world data, which are particularly necessary when studying patients who are underrepresented in clinical trials. Second, the absence of uniform screening for DM2 and the lack of information about the disease duration of DM2 or HF are also limitations. Third, in this study we did not assess the changes over time of treatment (HF and anti-diabetes) or glycemic control. Thus, we could not evaluate the influence of longitudinal changes in these parameters. Along this line and given the underuse of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists at the time of this study, we could not evaluate the influence of this pharmacological group on the present findings. Fourth, the exclusion of patients who died during the index hospitalization may have introduced some selection bias. Finally, the lack of homogenous assessment of parameters of right ventricular function and left diastolic function precluded a more detailed evaluation of the cardiac differences between sexes.
CONCLUSIONIn patients with HFpEF, DM2 confers a higher risk of mortality in women than in men. Further studies are needed to identify the mechanisms underlying these findings, the differential effect for men and women, and the optimal treatment strategies to improve outcomes in diabetic women with HFpEF.
FUNDINGThis work was supported in part by grants from: Sociedad Española de Cardiología: Investigación Clínica en Cardiología, Grant SEC 2015, CIBERCV 16/11/00420, 16/11/00403, FEDER and PIE15/00013.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
CONFLICTS OF INTERESTThe authors have no conflicts of interest to disclose.
- –
Diabetes and HFpEF are common comorbidities.
- –
Diabetes is a risk factor for the development of heart failure.
- –
There is evidence of sex-specific progression of diabetic cardiomyopathy.
- –
Diabetes worsens prognosis in women—but not men—with HFpEF.
- –
Excess risk among diabetic women with heart failure was found in all-cause mortality, cardiovascular mortality, and hospitalizations.