To compare the long-term results of direct oral anticoagulants (DOAC) vs vitamin K antagonists (VKA) in real-world-patients with nonvalvular atrial fibrillation (NVAF) in a nationwide, prospective study.
MethodsThe FANTASIIA registry prospectively included outpatients with AF anticoagulated with DOAC or VKA (per protocol, proportion of VKA and DOAC 4:1), consecutively recruited from June 2013 to October 2014 in Spain. The incidence of major events was analyzed and compared according to the anticoagulant treatment received.
ResultsA total of 2178 patients were included in the study (mean age 73.8±9.4 years), and 43.8% were women. Of these, 533 (24.5%) received DOAC and 1645 (75.5%) VKA. After a median follow up of 32.4 months, patients receiving DOAC vs those receiving VKA had lower rates of stroke—0.40 (95%CI, 0.17-0.97) vs 1.07 (95%CI,0.79-1.46) patients/y, P=.032—, severe bleedings—2.13 (95%CI, 1.45-3.13) vs 3.28 (95%CI, 2.75-3.93) patients/y; P = .044—, cardiovascular death—1.20 (95%CI, 0.72-1.99) vs 2.45 (95%CI, 2.00-3.00) patients/y; P = .009—, and all-cause death—3.77 (95%CI, 2.83-5.01) vs 5.54 (95%CI, 4.83-6.34) patients/y; P = .016—. In a modified Cox regression model by the Andersen-Gill method for multiple events, hazard ratios for patients receiving DOAC were: 0.42 (0.16-1.07) for stroke; 0.47 (0.20-1.16) for total embolisms; 0.76 (0.50-1.15) for severe bleedings; 0.67 (0.39-1.18) for cardiovascular death; 0.86 (0.62-1.19) for all-cause death, and 0.82 (0.64-1.05) for the combined event consisting of stroke, embolism, severe bleeding, and all-cause death.
ConclusionsCompared with VKA, DOAC is associated with a trend to a lower incidence of all major events, including death, in patients with NVAF in Spain.
Keywords
Nonvalvular atrial fibrillation (NVAF) is a common arrhythmia whose incidence and prevalence increase notably with age. In the adult population of Spain, it has an estimated prevalence of 4.4% (OFRECE study).1 Atrial fibrillation (AF) is not a benign arrhythmia, as morbidity and mortality are significantly increased in patients with this condition, particularly in terms of stroke and other systemic thromboembolic phenomena.2 Fortunately, this risk is considerably lowered by the use of oral anticoagulants, such as the classic vitamin K antagonists (VKAs).3 The last few years have witnessed the development of new direct oral anticoagulants (DOACs) (dabigatran, rivaroxaban, apixaban, and edoxaban) that limit these problems. Clinical trials comparing these drugs with warfarin in patients with NVAF have reported similar or superior efficacy in preventing stroke, with lower rates of severe bleeding, particularly intracranial bleeding, indicating greater safety.4–7 In addition, several meta-analyses have shown that DOACs can decrease mortality in NVAF patients.8 Based on these findings, clinical practice guidelines9,10 recommend these drugs as the anticoagulants of choice for NVAF patients with no contraindications, preferring them over VKAs. Various observational registries and studies using data from insurance companies in the United States have confirmed the safety and effectiveness of DOACs in real-world NVAF patients, with generally favorable results for DOACs vs VKAs.11–16
However, these studies have some methodological limitations and most of them compare DOACs with warfarin sodium, which is not the VKA commonly used in Spain. Furthermore, at the time the present study was designed and initiated, there were no real-world studies in Spain comparing these drugs. The aim of this prospective, observational, multicenter study was to compare the effectiveness and safety of DOACs vs VKAs (mainly acenocoumarol) in real-world patients with NVAF in Spain.
METHODSThe FANTASIIA study (atrial fibrillation: influence of the level and type of anticoagulation on the incidence of stroke and bleeding events) was designed and developed by the Research Agency of the Spanish Society of Cardiology, with the main objective stated above. The secondary aims were to analyze the quality of VKA anticoagulation and study the characteristics and clinical care related to NVAF in Spain. The study was approved by the Clinical Research Ethics Committee of San Juan Hospital in Alicante and met the requirements and standards of the Declaration of Helsinki and its subsequent amendments for research studies in humans, as well as the current data protection regulations in Spain. It is a nationwide, multicenter, observational study with a prospective follow-up, including consecutive NVAF patients who had been taking oral anticoagulants (DOACs or VKAs) on a stable basis for at least 6 months before enrollment and had provided informed consent for participation.
Inclusion and exclusion criteriaThe following inclusion criteria were applied: a) patients aged ≥ 18 years; b) diagnosis of NVAF (AF in the absence of a prosthetic heart valve and moderate/severe mitral stenosis); c) receiving oral anticoagulants on a continuous, stable basis for at least 6 months before enrollment; and d) provided written informed consent for participation. The exclusion criteria were as follows: a) age <18 years; b) any disorder that might affect the ability to grant written, informed consent; c) participation in a clinical trial at the time of possible inclusion; d) prosthetic heart valve or moderate/severe mitral stenosis; e) patient hospitalized at the time; f) unstable anticoagulation in the previous 6 months: that is, start and adjustment of VKA coagulation within the 6 months prior to inclusion, or discontinuation and restarting of VKA because of invasive procedures with a risk of bleeding (patients with dose changes or interruption of 1 or 2 doses due to an excessively high INR were eligible for inclusion); and g) unwilling to provide informed consent for participation.
Study design and developmentThe Research Agency of the Spanish Society of Cardiology appointed the scientific committee for the study, which was responsible for drafting the protocol and selecting the centers. One-hundred researchers (81 cardiologists and 19 internal medicine or primary care physicians) working in publically-funded health centers throughout Spain participated. Patient enrollment was conducted in outpatient consultations between June 2013 and October 2014. To “simulate” true DOAC use during that period in Spain, in each participating center, 1 patient receiving DOACs was included for every 4 patients receiving VKAs (predefined ratio in the protocol, 1:4 for DOACs and VKAs). Each investigator had to include the first 20 consecutive NVAF patients consulting who met the inclusion and exclusion criteria (first 4 with DOACs and first 16 with VKAs). During the enrollment visit, the baseline variables were recorded in an electronic data collection notebook. Subsequent yearly visits took place at 1, 2, and 3 years after the initial one, and the events that had occurred since the previous visit were recorded. If patients did not attend a visit, they were contacted by telephone or their medical history was consulted. The study was conducted in conditions of routine clinical practice, with no additional procedures or interventions.
Main outcome variable and sample sizeThe main effectiveness variable was the composite event stroke, other systemic embolism, major bleeding, or all-cause death (whichever was first). The time to the first event was used to construct Kaplan-Meier survival curves, whereas all events that occurred were included in the remaining analyses, even though there may have been several in the same patient. Differences between the 2 groups were also evaluated for the components of the composite variable and for cardiovascular death. Based on an estimated 3-year incidence for the main outcome variable of 18.5% in the VKA group and 13.5% in the DOAC group, a VKA:DOAC ratio of 4:1, and 5% of losses to follow-up, a sample size of 2175 patients would be needed with an alpha error of 5% and a beta error of 20%.
Statistical analysisQuantitative variables are expressed as the mean±standard deviation and qualitative variables as percentages. The Student t test was used for between-group comparisons among continuous variables and the chi-square test for qualitative variables. The cumulative incidence was calculated for the main outcome variable and secondary variables in the groups of interest; results are presented with their corresponding 95% confidence intervals (95%CI). Patients were analyzed by the treatment group they had been placed in at the initial visit. Kaplan-Meier survival curves were calculated and compared using the log-rank test. For the multivariate analysis, a modified Cox regression model was used according to the method proposed by Andersen-Gill for data with multiple events. Variables included in the model were age, sex, history of hypertension, history of diabetes, chronic obstructive pulmonary disease, renal failure, liver dysfunction, previous stroke, abbreviated Charlson index score,16 heart failure, coronary disease, previous major bleeding, AF type, European Heart Rhythm Association functional class, and use of antiarrhythmic therapy. A P value <.05 was considered statistically significant.
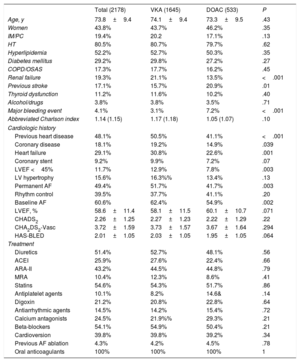
RESULTSBaseline characteristicsIn total, 2178 NVAF patients were included between June 2013 and October 2014, and 1956 of them had complete follow-up data. The characteristics of patients at the enrollment visit and the most important clinical background data are shown in Table 1. Mean age was 73.8±9.4 years, and 43.8% were women. Of the 2178 patients, 533 (24.5%) were taking DOACs and 1645 (75.5%) were taking VKAs. In the VKA group, 91% of patients were receiving acenocoumarol and 9% warfarin. In the DOAC group, 56.8% were taking dabigatran, 16.5% rivaroxaban, and 26.7% apixaban. The 2 groups were comparable for age, sex, cardiovascular risk factors, main comorbidities, and pharmacological treatment received. In comparison with the VKA group, a smaller percentage of patients receiving DOACs had previous heart failure (22.6% vs 30.8%; P=.001), coronary disease (14.9% vs 19.2%; P=.03), and renal failure (13.5% vs 21.1%; P <.001), and a higher percentage had previous stroke (20.9% vs 15.7%; P=.01) and major bleeding events (7.2% vs 3.1%; P <.001). There were no differences between the groups for the abbreviated Charlson, HAS-BLED, or CHA2DS2-VASc scores (Table 1).
Baseline characteristics of the patients in the total sample and in the 2 anticoagulant therapy groups (VKA and DOAC)
| Total (2178) | VKA (1645) | DOAC (533) | P | |
|---|---|---|---|---|
| Age, y | 73.8±9.4 | 74.1±9.4 | 73.3±9.5 | .43 |
| Women | 43.8% | 43.7% | 46.2% | .35 |
| IM/PC | 19.4% | 20.2 | 17.1% | .13 |
| HT | 80.5% | 80.7% | 79.7% | .62 |
| Hyperlipidemia | 52.2% | 52.7% | 50.3% | .35 |
| Diabetes mellitus | 29.2% | 29.8% | 27.2% | .27 |
| COPD/OSAS | 17.3% | 17.7% | 16.2% | .45 |
| Renal failure | 19.3% | 21.1% | 13.5% | <.001 |
| Previous stroke | 17.1% | 15.7% | 20.9% | .01 |
| Thyroid dysfunction | 11.2% | 11.6% | 10.2% | .40 |
| Alcohol/drugs | 3.8% | 3.8% | 3.5% | .71 |
| Major bleeding event | 4.1% | 3.1% | 7.2% | <.001 |
| Abbreviated Charlson index | 1.14 (1.15) | 1.17 (1.18) | 1.05 (1.07) | .10 |
| Cardiologic history | ||||
| Previous heart disease | 48.1% | 50.5% | 41.1% | <.001 |
| Coronary disease | 18.1% | 19.2% | 14.9% | .039 |
| Heart failure | 29.1% | 30.8% | 22.6% | .001 |
| Coronary stent | 9.2% | 9.9% | 7.2% | .07 |
| LVEF <45% | 11.7% | 12.9% | 7.8% | .003 |
| LV hypertrophy | 15.6% | 16.3%% | 13.4% | .13 |
| Permanent AF | 49.4% | 51.7% | 41.7% | .003 |
| Rhythm control | 39.5% | 37.7% | 41.1% | .20 |
| Baseline AF | 60.6% | 62.4% | 54.9% | .002 |
| LVEF, % | 58.6±11.4 | 58.1±11.5 | 60.1±10.7 | .071 |
| CHADS2 | 2.26±1.25 | 2.27±1.23 | 2.22±1.29 | .22 |
| CHA2DS2-Vasc | 3.72±1.59 | 3.73±1.57 | 3.67±1.64 | .294 |
| HAS-BLED | 2.01±1.05 | 2.03±1.05 | 1.95±1.05 | .064 |
| Treatment | ||||
| Diuretics | 51.4% | 52.7% | 48.1% | .56 |
| ACEI | 25.9% | 27.6% | 22.4% | .66 |
| ARA-II | 43.2% | 44.5% | 44.8% | .79 |
| MRA | 10.4% | 12.3% | 8.6% | .41 |
| Statins | 54.6% | 54.3% | 51.7% | .86 |
| Antiplatelet agents | 10.1% | 8.2% | 14.6& | .14 |
| Digoxin | 21.2% | 20.8% | 22.8% | .64 |
| Antiarrhythmic agents | 14.5% | 14.2% | 15.4% | .72 |
| Calcium antagonists | 24.5% | 21.9%% | 29.3% | .21 |
| Beta-blockers | 54.1% | 54.9% | 50.4% | .21 |
| Cardioversion | 39.8% | 39.8% | 39.2% | .34 |
| Previous AF ablation | 4.3% | 4.2% | 4.5% | .78 |
| Oral anticoagulants | 100% | 100% | 100% | 1 |
ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARA II, angiotensin II receptor antagonist; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; HT, hypertension; IM, internal medicine; LV, left ventricle; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; OSAS, obstructive sleep apnea syndrome; PC, primary care; VKA, vitamin K antagonist
Values are expressed as the mean±standard deviation, unless otherwise indicated.
Median follow-up was 32.4 months. All patients completed the first year of follow-up, 96.5% the second year, and 90% the third year. At 1 year of follow-up, 64.6% were receiving VKAs, 31.8% DOACs, and 3.6% no anticoagulants. At 2 years, the values were 57.5%, 39.2%, and 3.3%, respectively. At the end of follow-up, 51.3% of patients were taking VKAs, 44.1% DOACs, and 4.6% no anticoagulants. During the year when the study was designed (2013), average DOAC use over the total oral anticoagulant use in Spain was 5.4%, ranging from 3.6% in Navarre and La Rioja to 10.2% in Andalusia. In June 2018, the national average was 35.6%, ranging from 26.2% in Asturias to 56.8% in Cantabria. The percentage of time patients receiving VKAs were within the therapeutic range, evaluated with the Rosendaal method, was 61.43% (95%CI, 60.15%-62.71%) at the baseline visit, 62.08% (95%CI, 60.96%-63.20%) in the first year, 63.33% (95%CI, 62.03%-64.63%) in the second year, and 61.01% (95%CI, 59.31%-62.71%) in the third year.
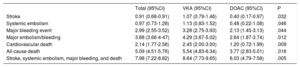
Overall incidence of events in the total seriesThe crude incidence rate per each 100 patients per year of the composite outcome variable and the various major events in the overall series is shown in Table 2. The incidence per 100 patients per year of the composite variable (stroke, other systemic embolism, major bleeding, or all-cause death) was 7.98: stroke 0.91, major bleeding 2.99, and all-cause mortality, 5.09. In the overall series, 19.68% of patients experienced the composite variable during follow-up: 2.30% stroke, 7.46% major bleeding, and 13.04% died during the study.
Crude event rate/100 patients/year in the total sample and in the VKA and DOAC groups
| Total (95%CI) | VKA (95%CI) | DOAC (95%CI) | P | |
|---|---|---|---|---|
| Stroke | 0.91 (0.68-0.91) | 1.07 (0.79-1.46) | 0.40 (0.17-0.97) | .032 |
| Systemic embolism | 0.97 (0.73-1.28) | 1.13 (0.83-1.52) | 0.48 (0.22-1.08) | .046 |
| Major bleeding event | 2.99 (2.55-3.52) | 3.28 (2.75-3.93) | 2.13 (1.45-3.13) | .044 |
| Major embolism/bleeding | 3.88 (3.66-4-47) | 4.29 (3.67-5.02) | 2.64 (1.87-3.74) | .012 |
| Cardiovascular death | 2.14 (1.77-2.58) | 2.45 (2.00-3.00) | 1.20 (0.72-1.99) | .009 |
| All-cause death | 5.09 (4.51-5.76) | 5.54 (4.83-6.34) | 3.77 (2.83-5.01) | .016 |
| Stroke, systemic embolism, major bleeding, and death | 7.98 (7.22-8.82) | 8.64 (7.73-9.65) | 6.03 (4.79-7.58) | .005 |
95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist
The unadjusted (crude) event rates per each 100 patients per year in the 2 groups are summarized Table 2. Patients receiving DOACs at the start of the study showed a significantly lower yearly incidence of all events than those receiving VKA, including the composite outcome variable (30% lower), stroke (62% lower), major bleeding (35% lower), cardiovascular death (51% lower), and all-cause death (32% lower).
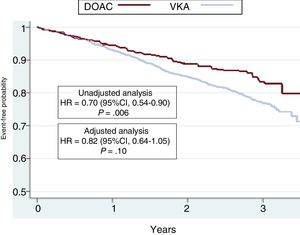
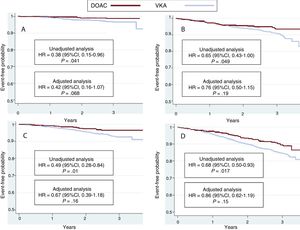
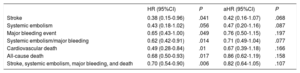
The hazard ratios (HRs) for all events are summarized in Table 3. The results were significantly in favor of the DOAC group in the unadjusted (crude) analysis. HR values after adjustment for potentially confounding variables for each event in the logistic regression model are also depicted in Table 3. There was a nonsignificant lower incidence of all events In DOAC-treated patients than in VKA-treated patients. HR values were 0.42 (95%CI, 0.16-1.07) for stroke (P=.06), 0.47 (95%CI, 0.20-1.16) for systemic embolism (P=.08), 0.76 (95%CI, 0.50-1.15) for major bleeding (P=.19), 0.67 (95%CI, 0.39-1.18) for cardiovascular death, 0.86 (95%CI, 0.62-1.19) for all-cause death, and 0.82 (95%CI, 0.64-1.05) for the composite outcome variable (stroke, embolism, major bleeding, and death) (P=.10). Kaplan-Meier survival curves for the composite outcome variable are shown in Figure 1, and curves for stroke, major bleeding, cardiovascular death, and all-cause death are shown in Figure 2 with the corresponding unadjusted and adjusted HRs.
Risk ratios for the various events between patients receiving DOACs and those taking VKAs, in the unadjusted and adjusted analyses (Cox regression models)
| HR (95%CI) | P | aHR (95%CI) | P | |
|---|---|---|---|---|
| Stroke | 0.38 (0.15-0.96) | .041 | 0.42 (0.16-1.07) | .068 |
| Systemic embolism | 0.43 (0.18-1.02) | .056 | 0.47 (0.20-1.16) | .087 |
| Major bleeding event | 0.65 (0.43-1.00) | .049 | 0.76 (0.50-1.15) | .197 |
| Systemic embolism/major bleeding | 0.62 (0.42-0.91) | .014 | 0.71 (0.49-1.04) | .077 |
| Cardiovascular death | 0.49 (0.28-0.84) | .01 | 0.67 (0.39-1.18) | .166 |
| All-cause death | 0.68 (0.50-0.93) | .017 | 0.86 (0.62-1.19) | .158 |
| Stroke, systemic embolism, major bleeding, and death | 0.70 (0.54-0.90) | .006 | 0.82 (0.64-1.05) | .107 |
95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; aHR, adjusted HR; VKA, vitamin K antagonist
Kaplan-Meier curves for the incidence of the composite outcome variable (stroke, systemic embolism, major bleeding, and death) in the 2 groups of patients. The results of the unadjusted and adjusted analyses are shown. 95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; VKA, vitamin K antagonist. gr1.
Kaplan-Meier curves for the incidence of stroke (A), major bleeding (B), cardiovascular death (C), and all-cause death (D) in the 2 groups of patients. The results of the unadjusted and adjusted analyses are shown. 95%CI, 95% confidence interval; DOAC, direct oral anticoagulant; HR, hazard ratio; VKA, vitamin K antagonist.
In addition to the advantages of DOACs related to their pharmacokinetic and pharmacodynamic properties (eg, fixed anticoagulant action, systemic monitoring of anticoagulation is not needed, and few interactions with other drugs, and foods), clinical trials investigating the 4 currently available DOACs4–7 and recently published real-world studies11–15 have reported efficacy similar to or higher than warfarin for stroke prevention, and greater safety in terms of severe bleeding events, in particular intracranial bleeding.4–7 Furthermore, a meta-analysis has even reported a reduction in mortality.8 Based on these results, certain clinical practice guidelines9,10 recommend DOACs as the anticoagulant agents of choice in patients with NVAF, preferring them over VKAs.
Nonetheless, the introduction of DOACs in Spain has been very slow, and the usage rates are much lower than those of neighboring countries.17 This situation is due to several factors. Among the most important may be the restrictions on DOAC prescription imposed by the therapeutic positioning report and the need to authorize its use by the autonomous communities.17 There are other possible reasons for this low utilization, such as poor control of the quality of VKA anticoagulation by physicians,18,19 and the absence of data from Spanish studies confirming the reproducibility of DOAC results in Spain. Several national studies have reported that anticoagulation is poor in around 50% of NVAF patients receiving VKAs, as they were found to be within the therapeutic range in the previous 6 months for less than 60% to 65% of the time,18,19 which is one of the allowed indications for switching to DOACs.17
Until the development of FANTASIIA, there were no multicenter studies comparing the overall effect of DOACs vs VKAs in clinical practice in Spain. Furthermore, the VKA most commonly prescribed in this country is not warfarin (the comparator used in all studies from other countries), which has important pharmacokinetic differences with respect to acenocoumarol (the VKA usually used in Spain). Hence, doubts may arise as to whether the results of these studies can be extrapolated to a setting such as Spain, where the most commonly used anticoagulant is acenocoumarol. FANTASIIA was specifically designed to provide a response to these uncertainties. The results of this study in NVAF patients (Figure 1, Figure 2, Table 2, Table 3) indicate a trend to greater effectiveness and safety of DOACs as a group compared with acenocoumarol in clinical practice in Spain. Patients receiving DOACs at the start of the study showed a statistically significant reduction in all the outcome events (stroke, systemic embolism, major bleeding, cardiovascular death, and all-cause death). Furthermore these reductions were of considerable magnitude in the unadjusted analysis, ranging from 32% for all-cause mortality to 62% for stroke (Table 3).
As could be expected,15 although this was a prospective study, its observational nature and setting of daily clinical practice led to differences in the baseline characteristics of the 2 patient groups (Table 1) that could have impacted the results. In patients receiving DOACs, there was a lower percentage of heart failure, coronary disease, and renal failure, and a higher percentage of previous stroke and major bleeding than in the VKA group. The embolic and hemorrhagic risk profile based on the HAS-BLED and CHA2DS2-VASc scores was, however, similar in the 2 groups, as well as the age, percentage of women, and treatments they were receiving (Table 1). After adjustment for potential confounding factors, a numerically important reduction in the incidence of all events was seen in favor of DOACs (reductions from 14% in mortality to 58% in stroke relative to VKAs) (Table 3).
As is the case of all studies performed in clinical practice,15 FANTASIIA has strengths and limitations. It is a cohort study with prospective follow-up and sample calculation based on the expected incidence of events in both treatment groups, which closely approached the actual results (at 3 years, 21% vs the expected 18% in the VKA group and 15.4% vs the expected 13.5% in the DOAC group). Furthermore, 91% of patients in the VKA group were taking acenocoumarol, which makes the findings applicable to patients in Spain. Last, VKA:DOAC sampling was at a 4:1 ratio to simulate the relative use of the 2 drug classes in Spain, where DOACs accounted for only 20% to 25% of the total of anticoagulants prescribed at the start of the study. FANTASIIA included a large number of patients (2178), there were few losses to follow-up, and more than 90% of patients had follow-up data. The limitations of the study are derived from its observational nature, as inclusion bias could not be completely eliminated, although it was minimized by the criterion of consecutive enrollment. Likewise, there were baseline differences between the groups, with a higher prevalence of previous heart disease and renal failure in the VKA group and stroke and bleeding in the DOAC group, which could have had (and in fact, did have) an influence on the difference in outcomes. In effect, the unadjusted analysis showed highly significant reductions in all outcome events in the DOAC group (Figure 1 and Figure 2), which decreased after adjustment for potential confounding factors. Nonetheless, a trend to a reduction in all outcome measures, both individual and combined, was maintained, with considerable numerical differences in favor of DOACs. Finally, the 4:1 allocation may have produced a certain over-representation or under-representation of patients, depending on the autonomous community where they were enrolled.
Another factor to consider when interpreting the results is the treatment changes during follow-up, which are known to occur in all studies with a lengthy follow-up.14,15 This is particularly true in observational studies, although it has also been seen in clinical trials (exemplified by the discussions regarding the recent CABANA AF ablation study results).20 At the start of the present study, 75.5% of patients were taking VKAs and 24.5%, DOACs. At the end of follow-up, 51.3% were receiving VKAs, 44.1% DOACs, and 4.6% no anticoagulant therapy. The analysis of outcome events was carried out according to the patients’ treatment group at the initial visit, but some events may have taken place while patients were receiving a different treatment from the initial one. This can occur in all observational studies performed in clinical practice and it does not affect the validity of the results. The analysis presented is conservative in the light of the treatment changes, as switches in the treatment group during follow-up (which occurred, although in a small percentage) tend to dilute the magnitude of an effect of treatment, if there is one. The data found are similar to those reported in studies performed in the United States, using information from insurance companies’ databases,11–13 which include a large number of patients but have considerable limitations, and data from European registries,14,15 which are more methodologically robust.
CONCLUSIONSFrom the clinical perspective, the findings of this study, in which the comparator drug was acenocoumarol, support the applicability to Spain of the results of trials and clinical practice studies performed in other countries. Despite the aforementioned limitations inherent to observational studies, the results indicate that compared with VKA therapy, DOAC use is associated with trends to a lower incidence of major AF-related events, including death, in NVAF patients in Spain. Hence, DOAC use should be increased in Spain to the levels seen in our neighboring countries to improve the prognosis of patients with this condition.20
FUNDINGThis study was funded by an unconditional grant from the Bristol-Myers-Squibb and Pfizer Alliance, Spain.
CONFLICTS OF INTERESTM. Anguita has received fees for lectures and consultancies from Pfizer, Bristol-Myers-Squibb, Daichii-Sankyo and Bayer. M. Ruiz has received fees for lectures and assistance to attend conferences held by Bayer, Boehringer-Ingelheim and Daiichi-Sankyo, and grants from Pfizer-Bristol and Instituto Carlos III. Á. Cequier has received fees for lectures and grants from Abbot, Biosense, Boston, Medtronic, Cordis, Biomens, Orbus Neich and the Spanish Society of Cardiology, and fees for lectures from Astra-Zeneca, Amgen, Bayer, Biotronik, Boehringer-Ingelheim and Daiichi-Sankyo, Ferrer, Sanofi, and Terumo. J. Muñiz has received fees for studies from the Spanish Society of Cardiology and for statistical analyses from Astellas Pharma. L. Badimón Maestro has received fees for lectures from Bayer and Pfizer, and grants from Astra-Zeneca. F. Marín has received fees for lectures and consultancies from Boehringer-Ingelheim, Daiichi-Sankyo and AF-NET, assistance for attending meetings held by Daichii-Sankyo, and grants from Pfizer-BMS and the Spanish Society of Cardiology.
- –
AF is a prevalent condition with a high risk of complications, which can be lowered with the use of oral anticoagulants. The new DOACs have shown similar or greater efficacy and safety than VKAs in clinical trials, meta-analyses, and observational registries performed outside of Spain, in which warfarin was the comparator drug. However, there have been no studies to date on DOAC use in real-world clinical practice in Spain, and above all, none with acenocoumarol as the comparator.
- –
The findings of this study, in which acenocoumarol was the comparator drug, support the applicability to Spain of the results of trials and clinical practice studies performed in other countries. Despite the limitations inherent to observational studies, the results obtained indicate that in comparison with VKAs, DOAC use is associated with trends to a lower incidence of major AF-related events in NVAF patients. Greater use of these drugs could improve the prognosis of patients with NVAF in Spain.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2019.02.021