Outcomes of patients undergoing percutaneous intervention for drug-eluting stent (DES) restenosis are poorer than those in patients with bare-metal stent restenosis. It is unknown if this is related to the presence of polymer coating. We sought to compare outcomes after interventions for in-stent restenosis (ISR) of polymer-free DES vs durable polymer DES.
MethodsPatients enrolled in the ISAR-TEST 5 randomized trial who underwent repeat percutaneous intervention for ISR during follow-up were included. Angiographic outcomes at 6 to 8 months and clinical outcomes at 2 years were analyzed and compared between 2 groups according to whether the restenosed stent was a polymer-free or a durable polymer DES. Multivariate analysis was used to adjust for differences between groups.
ResultsA total of 326 patients with ISR were included: 220 with ISR in polymer-free DES and 106 with ISR in durable polymer DES. Angiographic follow-up was available for 83.4% of patients. No difference was observed in recurrent binary restenosis between the 2 groups (31.7% vs 27.0%; P=.38; Padjusted=.29). At 2 years, the composite of death, myocardial infarction, or repeat target lesion revascularization were similar between the 2 groups (35.7% vs 34.0%; HR=1.04, 95%CI, 0.70-1.55; P=.83; Padjusted=.79). The rate of repeat target lesion revascularization was also similar in the 2 groups (29.8% vs 31.5%; HR=0.91, 95%CI, 0.60-1.39; P=.68; Padjusted=.62).
ConclusionsIn patients undergoing reintervention for DES-ISR, we found no evidence of differences in outcomes according to whether the restenosed stent was a polymer-free or durable polymer DES.
Keywords
Patients treated for coronary in-stent restenosis (ISR) remain at high risk for adverse events during follow-up.1 The most effective treatment strategies are angioplasty with drug-coated balloon and repeat stenting with second-generation drug-eluting stents (DES).2,3 However, for reasons that remain poorly understood, patients treated for ISR in DES have higher adverse event rates than those treated for ISR in bare-metal stents.4,5
The efficacy of DES is largely dependent on the controlled release of the active drug from the stent backbone.6,7 Most DES approved for clinical use have polymer coatings, which control the drug release kinetic.8 However, polymer coatings have been implicated as a cause of inflammatory response of the artery following stenting.9,10 Such inflammatory responses can induce delayed arterial healing and may play a role in the development of both neointimal hyperplasia and in-stent neoatherosclerosis.11
Polymer-free DES is a newer generation stent technology that may overcome the disadvantages of polymer coating of durable polymer DES.8 However, it is not known whether ISR in polymer-free DES responds differently to treatment compared with ISR in durable polymer DES. We investigated patients with ISR in the ISAR-TEST 5 randomized trial12 and sought to compare outcomes after intervention for ISR in polymer-free vs durable polymer DES.
METHODSStudy population and study protocolISAR-TEST 5 was a randomized, noninferiority trial, in which 3002 patients were assigned in a 2:1 treatment allocation to receive either polymer-free DES (dual drug sirolimus- and probucol-eluting stent, backbone Yukon stent, Translumina, Hechingen, Germany; device iterated as Coroflex Isar stent, B. Braun Melsungen, Melsungen, Germany) or a second-generation durable polymer DES (zotarolimus-eluting stent, Resolute, Medtronic Vascular, Santa Rosa, CA, United States).12 Patients who were enrolled in the ISAR-TEST 5 trial and received percutaneous coronary intervention for ISR within 2 years after their index procedures were included in this study. We excluded patients who were treated with percutaneous intervention for stent thrombosis. The main trial identifier is NCT00598533.
Procedures and antithrombotic medicationsDuring the ISR procedure, patients were treated with repeat stenting with first generation DES, repeat stenting with second-generation DES, balloon angioplasty, or drug-coated balloon angioplasty. First generation DES comprised durable polymer sirolimus-eluting stents (Cypher, Cordis, Warren, NJ, United States), polymer-free sirolimus-eluting stents (Yukon, Translumina), durable polymer paclitaxel-eluting stents (Taxus, Boston Scientific, Natick, MA, United States) and durable polymer zotarolimus-eluting stents (Endeavor, Medtronic). Second-generation DES included durable polymer everolimus-eluting stents (Xience, Abbott Vascular, Santa Clara, CA, United States), durable polymer zotarolimus-eluting stents (Resolute, Medtronic), biodegradable polymer sirolimus-eluting stents (Yukon, Translumina), and sirolimus and probucol-eluting stents (Yukon, Translumina and Coroflex Isar, B. Braun Melsungen).
After the intervention, all patients were prescribed aspirin indefinitely, and clopidogrel 75mg/d or ticagrelor 90mg twice/d, or prasugrel 5 to 10mg/d for at least 6 months.
Follow-upFollow-up angiography was scheduled 6 to 8 months after the repeat intervention for treatment of ISR, as part of routine practice in patients treated for ISR at the 2 participating institutions. Quantitative coronary angiographic analysis at pre- and postinterventions and at follow-up was carried out with a validated automated edge-detection system (QAngioXA version 7.3, Medis Medical Imaging Systems) in an angiographic core laboratory (ISAResearch Center, Munich, Germany). Clinical follow-up was performed either by telephone contact, structured follow-up letter, or office visit at 1 month and at 1 and 2 years after the repeat intervention. All clinical events were adjudicated and classified by independent adjudicators.
Endpoints and definitionsThe primary endpoint of interest in this study was the composite of all-cause death, myocardial infarction, or target lesion revascularization (TLR) 2 years after ISR treatment. Secondary endpoints of interest were binary restenosis rate and late luminal loss at angiographic follow-up, and all-cause death, myocardial infarction, TLR, and definite/probable stent thrombosis at 2 years. The definitions of the individual endpoints were consistent with the original trial protocol.12
Statistical analysisContinuous data are presented as mean (± standard deviation) or median (25th-75th percentiles). Categorical data are presented as counts and proportions (%). Differences between groups were checked using the Student's t or Wilcoxon rank sum test for continuous variables and the chi-square or Fisher exact test for categorical variables. Event-free survival was assessed using the Kaplan-Meier method. Hazard ratios with accompanying 95% confidence intervals (95%CI) were estimated from univariate Cox proportional hazards models. P values <.05 were considered statistically significant. Multivariate analysis was performed for the primary endpoint of interest and for TLR in order to adjust for differences in baseline characteristics and ISR treatments between groups. Cox proportional hazards models were used for clinical outcomes based on survival analysis; logistic regression analysis was used for binary restenosis. In view of the number of patients included in the study, we restricted inclusion to all variables with a P <.1 in the univariate analysis. Statistical software S-PLUS, version 4.5 (S-PLUS, Insightful Corp, Seattle, United States) was used for the analyses.
RESULTSPatients, lesions, and procedural characteristicsOf a total of 3002 patients enrolled in the ISAR-TEST 5 trial, 326 underwent repeat percutaneous coronary intervention for ISR within 2 years after the index intervention. Due to the initial 2:1 allocation of polymer-free DES and durable polymer DES, we had 220 ISR patients with polymer-free DES and 106 ISR patients with durable polymer DES.
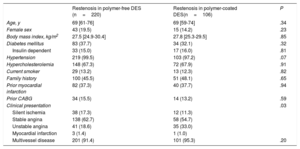
The baseline characteristics of patients presenting with ISR are shown in Table 1. There were no significant differences between groups with the exception of the prevalence of acute coronary syndrome at presentation, which was lower in the polymer-free DES-ISR group than in the durable polymer DES-ISR group (20.0% vs 34.0%; P=.006).
Baseline clinical characteristics
| Restenosis in polymer-free DES (n=220) | Restenosis in polymer-coated DES(n=106) | P | |
|---|---|---|---|
| Age, y | 69 [61-76] | 69 [59-74] | .34 |
| Female sex | 43 (19.5) | 15 (14.2) | .23 |
| Body mass index, kg/m2 | 27.5 [24.9-30.4] | 27.8 [25.3-29.5] | .85 |
| Diabetes mellitus | 83 (37.7) | 34 (32.1) | .32 |
| Insulin dependent | 33 (15.0) | 17 (16.0) | .81 |
| Hypertension | 219 (99.5) | 103 (97.2) | .07 |
| Hypercholesterolemia | 148 (67.3) | 72 (67.9) | .91 |
| Current smoker | 29 (13.2) | 13 (12.3) | .82 |
| Family history | 100 (45.5) | 51 (48.1) | .65 |
| Prior myocardial infarction | 82 (37.3) | 40 (37.7) | .94 |
| Prior CABG | 34 (15.5) | 14 (13.2) | .59 |
| Clinical presentation | .03 | ||
| Silent ischemia | 38 (17.3) | 12 (11.3) | |
| Stable angina | 138 (62.7) | 58 (54.7) | |
| Unstable angina | 41 (18.6) | 35 (33.0) | |
| Myocardial infarction | 3 (1.4) | 1 (1.0) | |
| Multivessel disease | 201 (91.4) | 101 (95.3) | .20 |
CABG, coronary artery bypass surgery; DES, drug-eluting stent.
Data are shown as median [interquartile range] or No. (%)
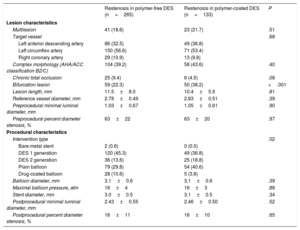
The baseline characteristics of lesions with ISR are shown in Table 2. A total of 398 ISR lesions were treated with repeat percutaneous coronary intervention (polymer-free DES-ISR, n=265; durable polymer DES-ISR, n=133). Lesion characteristics were generally well balanced between the 2 groups. The prevalence of bifurcation lesions was lower in the polymer-free DES-ISR group than that in the durable polymer DES-ISR group (22.3% vs 38.2%; P <.001). For treatment of ISR, overall differences were observed between the groups in the device used (P=.017), as shown in Table 2.
Lesion and procedural characteristics
| Restenosis in polymer-free DES (n=265) | Restenosis in polymer-coated DES (n=133) | P | |
|---|---|---|---|
| Lesion characteristics | |||
| Multilesion | 41 (18.6) | 23 (21.7) | .51 |
| Target vessel | .68 | ||
| Left anterior descending artery | 86 (32.5) | 49 (36.8) | |
| Left circumflex artery | 150 (56.6) | 71 (53.4) | |
| Right coronary artery | 29 (10.9) | 13 (9.8) | |
| Complex morphology (AHA/ACC classification B2/C) | 104 (39.2) | 58 (43.6) | .40 |
| Chronic total occlusion | 25 (9.4) | 6 (4.5) | .08 |
| Bifurcation lesion | 59 (22.3) | 50 (38.2) | <.001 |
| Lesion length, mm | 11.5±8.0 | 10.4±5.9 | .81 |
| Reference vessel diameter, mm | 2.78±0.49 | 2.83±0.51 | .39 |
| Preprocedural minimal luminal diameter, mm | 1.03±0.67 | 1.05±0.61 | .90 |
| Preprocedural percent diameter stenosis, % | 63±22 | 63±20 | .97 |
| Procedural characteristics | |||
| Intervention type | .02 | ||
| Bare-metal stent | 2 (0.6) | 0 (0.0) | |
| DES 1 generation | 120 (45.3) | 49 (36.8) | |
| DES 2 generation | 36 (13.6) | 25 (18.8) | |
| Plain balloon | 79 (29.8) | 54 (40.6) | |
| Drug-coated balloon | 28 (10.6) | 5 (3.8) | |
| Balloon diameter, mm | 3.1±0.6 | 3.1±0.6 | .39 |
| Maximal balloon pressure, atm | 16±4 | 16±3 | .88 |
| Stent diameter, mm | 3.0±0.5 | 3.1±0.5 | .34 |
| Postprocedural minimal luminal diameter, mm | 2.43±0.55 | 2.46±0.50 | .52 |
| Postprocedural percent diameter stenosis, % | 16±11 | 16±10 | .65 |
AHA/ACC, American Heart Association/American College of Cardiology; DES, drug-eluting stent.
Data are shown as mean±standard deviation or No. (%).
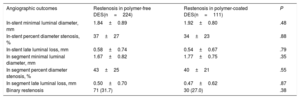
A total of 272 patients (83.4%) underwent angiographic follow-up. In-stent late luminal loss was 0.58±0.74mm in the polymer-free DES-ISR group and 0.54±0.67mm in the durable polymer DES-ISR group (P=.79) (Table 3). Binary restenosis was observed in 31.7% in the polymer-free DES-ISR group and in 27.0% in the durable polymer DES-ISR group (P=.38; Padjusted=.29).
Angiographic outcomes
| Angiographic outcomes | Restenosis in polymer-free DES(n=224) | Restenosis in polymer-coated DES(n=111) | P |
|---|---|---|---|
| In-stent minimal luminal diameter, mm | 1.84±0.89 | 1.92±0.80 | .48 |
| In-stent percent diameter stenosis, % | 37±27 | 34±23 | .88 |
| In-stent late luminal loss, mm | 0.58±0.74 | 0.54±0.67 | .79 |
| In segment minimal luminal diameter, mm | 1.67±0.82 | 1.77±0.75 | .35 |
| In segment percent diameter stenosis, % | 43±25 | 40±21 | .55 |
| In segment late luminal loss, mm | 0.50±0.70 | 0.47±0.62 | .87 |
| Binary restenosis | 71 (31.7) | 30 (27.0) | .38 |
DES, drug-eluting stent.
Data are shown as mean±standard deviation or No. (%).
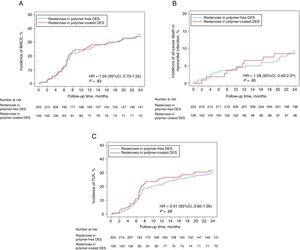
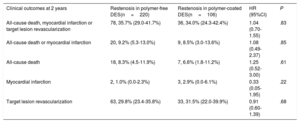
Two-year clinical outcomes are shown in Table 4. There was no significant difference in the occurrence of the primary composite endpoint at 2 years between the polymer-free DES-ISR and the durable polymer DES-ISR groups (35.7% vs 34.0%, hazard ratio [HR]=1.04, 95% confidence interval [95%CI], 0.70-1.55; Punadjusted=.83) (Figure 1A). Using multivariate analysis, we found no difference in clinical outcomes after 2 years when adjustment was made for differences in baseline characteristics and ISR treatment types (Padjusted=.79). In a sensitivity analysis comparing the primary endpoint of interest in patients in both groups treated with either drug-coated balloon or second-generation DES, we found no difference between the 2 groups (34.0% vs 28.0%, HR=1.31, 95%CI, 0.76-3.17; P=.54).
Clinical outcomes
| Clinical outcomes at 2 years | Restenosis in polymer-free DES(n=220) | Restenosis in polymer-coated DES(n=106) | HR (95%CI) | P |
|---|---|---|---|---|
| All-cause death, myocardial infarction or target lesion revascularization | 78, 35.7% (29.0-41.7%) | 36, 34.0% (24.3-42.4%) | 1.04 (0.70-1.55) | .83 |
| All-cause death or myocardial infarction | 20, 9.2% (5.3-13.0%) | 9, 8.5% (3.0-13.6%) | 1.08 (0.49-2.37) | .85 |
| All-cause death | 18, 8.3% (4.5-11.9%) | 7, 6.6% (1.8-11.2%) | 1.25 (0.52-3.00) | .61 |
| Myocardial infarction | 2, 1.0% (0.0-2.3%) | 3, 2.9% (0.0-6.1%) | 0.33 (0.05-1.95) | .22 |
| Target lesion revascularization | 63, 29.8% (23.4-35.8%) | 33, 31.5% (22.0-39.9%) | 0.91 (0.60-1.39) | .68 |
95%CI, 95% confidence interval; DES, drug-eluting stents; HR, hazard ratio.
Data are shown as No., % (95%CI); rates are estimated by Kaplan-Meier method; HR and P values were calculated by Cox proportional hazard methods.
Composite outcomes of all-cause death, myocardial infarction, and TLR. Time to event curve in cumulative incidences of MACE (A), all-cause death or myocardial infarction (B), and TLR (C). 95%CI, 95% confidence interval; DES, drug-eluting stents; HR, hazard ratio; MACE, major adverse cardiac events; TLR, target lesion revascularization. HR and P values are derived from Cox proportional hazard methods.
Individual component rates of the primary endpoint were similar between the 2 groups: all-cause death, 8.3% vs 6.6% (HR=1.25, 95%CI, 0.52-3.00; P=.61) and myocardial infarction, 1.0% vs 2.9% (HR=0.33, 95%CI, 0.05-1.95; P=.22) (Figure 1B). TLR was also similar between the groups: 29.8% vs 31.5% (HR=0.91, 95%CI, 0.60-1.39; Punadjusted=.68; Padjusted=.62) (Figure 1C). The median time from index intervention to TLR in both groups was similar: 211 [190-269] days vs 204 [166-269] days (P=.17).
No cases of stent thrombosis were observed.
DISCUSSIONWe aimed to investigate whether the presence or absence of a polymer coating on the original restenosed DES in patients with DES-ISR might impact patient outcomes after reintervention for ISR. The principal findings, following repeat interventions for ISR, were that there was no difference in the rate of binary angiographic restenosis between the polymer-free DES and the durable polymer DES groups. Moreover, after a follow-up period of 2 years, there was no difference in the composite clinical endpoint of all-cause death, myocardial infarction, TLR, or in the rate of repeat revascularization following repeat interventions for ISR in polymer-free DES compared with durable polymer DES.
Despite the overall high efficacy of DES, treatment of DES restenosis is more challenging than treatment of bare-metal stent restenosis. Data from randomized trials and registries show that DES-ISR compared with bare-metal stent ISR is associated with a higher risk of recurrent restenosis, requirement for repeat revascularization, and subsequent major adverse cardiac events.5,13–15 Moreover, a considerably higher rate of late re-restenosis was reported in patients treated with drug-coated balloon for DES restenosis compared with bare-metal stent restenosis.16
The underlying reasons for outcome differences after intervention for DES-ISR vs bare-metal stent ISR remain unknown. It is possible that the pathophysiology of ISR is different. For example, neoatherosclerosis may be a more frequent cause of DES-ISR and might be associated with worse outcomes.11 Another possible explanation is that patients who develop DES-ISR may have higher rates of resistance or hyporesponsiveness to antirestenotic drugs in general or sirolimus analogs in particular.5 However, it is also conceivable that long-term inflammatory reactions against the polymer coating on the originally implanted stent might impact outcomes following repeat intervention for ISR.
Polymer-free stent technology was developed with the aim of abolishing polymer-related persistent inflammatory responses, which may drive delayed arterial healing and neoatherosclerosis after stenting. Although early generation devices were inferior to durable polymer DES,6 technologically improved polymer-free DES demonstrated noninferiority to current-generation DES in short-term angiographic12,17 and longer-term clinical outcomes.18 Moreover, encouraging results were seen with polymer-free DES in a large-scale trial enrolling patients with high bleeding risk.19
The main finding of our study was that we found no evidence of outcome differences based on whether the restenotic DES were polymer-free or polymer-coated. To the best of our knowledge, this is the first study comparing the outcomes of patients treated for ISR in polymer-free vs durable polymer stents. Moreover, the rate of angiographic follow-up after repeat intervention was high (83.4%), and accordingly, the findings in relation to angiographic outcomes are likely to be robust.20 In addition, patients developing restenosis while using polymer-free and polymer-coated DES were well matched at the time of original stent implantation since treatment allocation was random. The observations should be interpreted in light of the fact that the polymer-free and permanent polymer stents studied differed in components other than the presence or absence of a polymer coating (eg, stent backbone, type of drug eluted), although this is unavoidable when comparing commercially available stents that combine specific stent components in a single device.
A number of reasons could explain the absence of differences observed between the treatment groups. First and foremost, the type of restenotic DES, whether polymer-free or polymer-coated, may not strongly influence outcomes subsequent to ISR treatment. Second, our study is observational and the existence of some differences between the treatment groups at the time of ISR treatment may have obscured any true effect. Third, the devices used to treat the 2 groups at the time of presentation with ISR were somewhat different. Indeed, although the proportion of patients treated with repeat DES stenting was similar, more patients with polymer-free DES had angioplasty with a drug-coated balloon than with a plain balloon. As the operators performing the repeat procedure were not blinded to the type of underlying DES, we cannot exclude the risk of treatment selection bias. However, multivariate analysis adjusted for different treatment types including first generation DES, second-generation DES, balloon angioplasty, or drug-coated balloon angioplasty, showed no differences. Finally, our study did not have sufficient statistical power to detect a difference in clinical outcomes. We estimated that a sample size of 326 patients with an event rate of 35%, a sampling ratio of 2, and an alpha of 5% would have 62% power to detect a difference of 20% between the treatment groups. Accordingly, a study using a larger number of patients may be required to detect existing differences.
CONCLUSIONSThe clinical and angiographic outcomes after treatment of polymer-free sirolimus- and probucol-eluting stents ISR were similar to those after treatment of durable polymer based zotarolimus-eluting stents ISR.
FUNDINGThis research was supported in part by the Bavarian Research Foundation (BFS-ISAR Aktenzeichen AZ: 504/02 and BFS-DES Aktenzeichen AZ: 668/05) and by the European Union FP7 (PRESTIGE 260309).
CONFLICTS OF INTERESTM.J. reports being a consultant for Biotronik and Orbus Neich and receiving research support from Biotronik and Orbus Neich. R.A.B. reports personal fees from B. Braun Melsungen AG, Biotronik, Boston Scientific and Micell Technologies as well as grants to the institution from Boston Scientific and Celonova Biosciences outside the submitted work.
- –
Several studies have shown that the outcomes of patients treated for DES restenosis are poorer than those of patients treated for bare-metal stent restenosis.
- –
Though the reasons for this difference are unknown, it may be related to the adverse effect of polymer coatings, which remain on the stent backbone long after their useful function (drug-elution) is served.
- –
We analyzed patients enrolled in a large-scale randomized clinical trial comparing polymer-free and durable polymer DES who presented with restenosis and compared outcomes after intervention for restenosis according to the original stent type.
- –
A high proportion of patients undergoing reintervention for DES-ISR underwent repeat angiography (83.4%).
- –
No difference was observed in recurrent binary restenosis according to whether the restenosed stent was polymer-free or had a durable polymer coating.
- –
The rate of adverse clinical events during follow-up was also comparable among patients treated for restenosis in polymer-free and durable polymer DES, even after adjustment for differences in treatment received.
The authors wish to acknowledge the contribution ISAR-TEST 5 trial Investigators.