To assess compliance with treatment inhibit the renin-angiotensin system (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) in uncontrolled hypertension in patients at high cardiovascular risk.
MethodsProspective, longitudinal, multicenter study, carried out in 102 Spanish primary care centers. We included 808 uncontrolled hypertensive patients treated with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers who were at high vascular risk; 4 visits were conducted: baseline and 1, 3, and 6 months later. Compliance was measured by electronic monitors. We calculated the mean percentage compliance, the overall percentage of compliers, once-daily compliers, compliers with the prescribed time frame, and antihypertensive coverage. We considered a patient to be a complier when the percentage compliance was 80%-100%.
ResultsIn all, 701 patients completed the study (mean age, 63.7 [11.1] years). The systolic and diastolic blood pressures decreased significantly (P<.0001) to 18.8mmHg and 9.8 mmHg, respectively. The control rate was 70% (95% confidence interval, 65.6%-74.4%) (P=.0001). The rate of control was significantly higher among compliers than noncompliers (P<.05). The mean percentage of doses taken was 87.9% (95% confidence interval, 84.8%-91%) and the mean therapeutic coverage was 82.4% (95% confidence interval, 78.7%-86.1%). Overall, 73.3% of the patients were compliers (95% confidence interval, 69%-77.6%), 52.8% (95% confidence interval, 48%-57.6%) were once-daily compliers, and 46.5% (95% confidence interval, 41.9%-51.1%) complied with the prescribed time frame. Noncompliance was associated with a higher number of drugs prescribed (P<.001).
ConclusionsIn hypertensive patients at high vascular risk, the rate of therapeutic noncompliance was very high, mainly when they took 5 or more pills daily.
Keywords
.
IntroductionTreatment compliance is considered to be “the extent to which the patient accepts the norms or advice provided by the physician or health care staff, not only with respect to recommended habits or lifestyle, but to the prescribed drug therapy itself, expressed as the degree of coincidence between the guidelines set forth by the professional, based on a well-reasoned decision, and their observance on the part of the patient”.1
A number of studies have demonstrated the negative impact of noncompliance with antihypertensive therapy on morbidity and mortality and on health care costs,2, 3 and it can be expected to have a greater effect in patients at high vascular risk.
The rate of control of hypertension (HT) in hypertensive patients in Spain is around 35% to 40%4 and reaches 60% among patients with ischemic heart disease.5 One of the major causes of the failure to achieve control is noncompliance with the drug therapy, which has a prevalence of 45%,6 and the study of this lack of success in different subgroups of patients should provide relevant information.
Most of the studies that assess compliance have involved the evaluation of patients with mild to moderate HT and at low vascular risk, who are usually taking only one antihypertensive agent.4 In Spain, only the ETECUM study has assessed compliance with a specific antihypertensive drug.7
There are no studies that assess compliance with different antihypertensive agents or in patients at high vascular risk, and the prevalence of the various patterns of noncompliance in these patients is unknown. Thus, we consider it important to study these aspects, with the aim of modifying them.
Our objective is to assess compliance with treatment to inhibit the renin-angiotensin system (angiotensin-converting enzyme [ACE] inhibitors or angiotensin receptor blockers [ARB]) with drug therapy for uncontrolled HT in patients at high vascular risk, measured with electronic monitors (the medication event monitoring system [MEMS]). The relevance of this study is substantiated by the importance of the early achievement of early blood pressure (BP) control to reduce cardiovascular morbidity and mortality in patients at high cardiovascular risk.8
Methods Type of Study and Calculation of the Sample SizeWe used a multicenter, longitudinal, prospective design to study 808 patients diagnosed as having uncontrolled HT according to the criteria of the 2007 European Society of Hypertension/European Society of Cardiology (ESH/ESC) consensus guidelines, developed in 102 Spanish primary care centers.
To determine the sample size for studies that obtain proportions as the major outcome measures, the following formula was employed9:
where n is the number of individuals needed; Zα=0.01, corresponding to a value of 2.576; e=5%, as the desired accuracy; and P=.50 and q=0.50, as maximum uncertainty. The sample size resulting from the calculations was 664 patients. An additional 18% was added in view of possible dropouts, for a final total of 808 patients. Initiation and Duration of the Study. Investigators
The duration of the study was 12 months (from March 2010 to February 2011). The patients were enrolled over a 4-month period and the mean follow-up was 6 months.
A total of 134 investigators participated and each had to select 6 patients by means of probability proportional to size sampling based on the number of physicians from each Spanish province. The centers in each province were selected in proportion to their number.
The patients were selected consecutively among individuals in whom uncontrolled HT was detected during a visit to the office of his or her physician.
Inclusion CriteriaThe study involved patients over the age of 18 years who had been diagnosed as having HT (according to the 2007 ESH/ESC criteria), were receiving antihypertensive therapy at least 3 months prior to the initiation of the study, in whom the disease was not controlled (diastolic blood pressure [DBP] of 140mmHg or over and/or systolic blood pressure [SBP] of 90mmHg or over), whose antihypertensive therapy was going to be modified, and were at high vascular risk (prior acute myocardial infarction or coronary artery disease, peripheral vascular disease, stroke or with type 2 diabetes mellitus plus an associated cardiovascular risk factor). The patients also had to give their written informed consent and be receiving treatment with an ACE inhibitor or an ARB.
Exclusion CriteriaHypertensive patients were excluded if they had secondary HT, were pregnant or breastfeeding, had some disease that the investigator considered could interfere with the course of the study, were participating in other research studies, or were living with someone who was taking the same antihypertensive agent.
Criteria for WithdrawalThe patients could withdraw from the study on their own decision, but hospital admission, previous noncompliance that justified not modifying the treatment, loss of data, or potentially unreliable data were also reasons for being excluded once the study was underway.
Main MeasurementsWe determined BP, weight, height, and waist circumference (WC). BP was measured according to the recommendations of the 2005 Spanish HT guidelines. Compliance with antihypertensive drug therapy was measured using the MEMS (Aardex, Switzerland), a well validated method. The percentage compliance (PC) was calculated according to the formula:
PC=total number of pills presumably consumed/total number of pills that should have been consumed×100
We considered the final PC for each hypertensive patient in the study to be the cumulative PC at the end of follow-up (at the conclusion of the last visit or when the patient withdrew or was excluded).
Work PlanThe patients made 4 visits, which included the enrollment visit, 2 follow-up visits, and the final visit. The enrollment visit was used to: a) confirm the inclusion and exclusion criteria; b) inform the patient orally and in writing and receive the patient's signed informed consent; c) record the medical history; d) measure weight, height and WC, and take BP twice in the same arm, and e) to give the patient a MEMS and to modify his or her antihypertensive therapy, prescribing an ACE inhibitor or an ARB, for the first time in some cases, in order to modify the previously prescribed dose or change to a different drug. These drugs were chosen because of their known efficacy in hypertensive patients at high vascular risk and to make pill counting more uniform and the results concerning compliance more representative. The patient acquired the drugs in the pharmacy and gave them to the investigator, who took the blister cards out of the boxes, separated the individual pills, leaving each blister intact, and introduced them into the MEMS. All the antihypertensive agents (ACE inhibitors, ARB, and others) were included. The patients were told how the MEMS functioned and were shown how to use it.
The follow-up visits took place at 4 and 12 weeks. BP was measured twice, and the weight and WC were recorded. If the patient had not achieved the therapeutic objectives with regard to BP control, the investigator could modify the antihypertensive treatment according to his or her criteria.
The final visit took place at 26 weeks, with a format similar to that of the follow-up visits. The MEMS were returned to the investigator and sent to the coordinating center, where they were analyzed by means of a reader and a specific software program.
Variables AnalyzedThe variables were age and sex, the total number of subjects, dropouts and the causes, number of diseases recorded, number of medications consumed, vascular risk factors, mean body mass index (BMI) and mean WC. The mean office BP (SBP and DBP) and the degree of HT control (mean SBP and DBP less than 140mmHg and less than 90mmHg, respectively) in the follow-up and final visits were calculated. We also calculated the mean PC and the final percentage of compliers (main variable), the percentage of days on which a subject had taken at least 1 once-a-day pill, the percentage of doses taken within the recommended time frame (from 8:00 to 9:00), and the therapeutic coverage or time during which the patient was covered by an antihypertensive agent, assuming a 24-h effect of said drug.
A patient was considered to be a complier if the PC was >80% and a noncomplier if it was lower. We assessed the different patterns of noncompliance defined in the literature.10
Compliers were classified as a) absolute compliers, PC of 100%; b) disguised compliers, PC>80% and daily PC<80%; c) compliers with sporadic noncompliance, PC greater than 80% and less than 100%, and d) overcompliant, PC greater than 100%.
Noncompliers were classified as: a) absolute noncompliers, PC <50%; b) partial noncompliers, PC 50% to 80%, and c) dropouts, those who stopped taking the medication permanently.
Other patterns of compliance or noncompliance were: a) foreseeable noncompliance due to a constant pattern of noncompliance; b) drug holidays, periods of at least 3 consecutive days during which the medication is not taken; c) white-coat compliance, intake of the medication on the days immediately preceding and following the visit to the physician, with incompliance in between; d) noncompliance regarding timing, failure to take the medication between 7:00 and 9:00, and e) mixed noncompliance, where there were two or more patterns of noncompliance.
The study was carried out in accordance with the ethical guidelines of the Declaration of Helsinki and was authorized by the Research Committee of Hospital Juan Ramón Jiménez in Huelva, Spain.
Statistical AnalysisA descriptive statistical analysis was performed. In the bivariate analysis, we used the χ2 and Student t test for the comparison of qualitative and quantitative variables, respectively. The multivariate analysis was carried out with backward stepwise logistic regression. The variables of the compliers and noncompliers were compared. A P value greater than .05 was considered to indicate statistical significance. The 95% confidence intervals (95%CI) were calculated. The Paradox 3.5 database and SPSS PC+s15 software package were employed.
We assessed the intervention carried out between the first month and the end of the study (intervention: follow-up, treatment changes according to clinical practice guidelines, use of the MEMS, and news bulletins concerning the study for the investigators), determining whether the differences observed in HT control were clinically relevant. This relevance was estimated on the basis of its indicators, such as the calculation of the absolute risk reduction, relative risk reduction (RRR), number of patients needed to treat with the intervention to avoid poor HT control, and relative risk (RR) as a measure of association.9
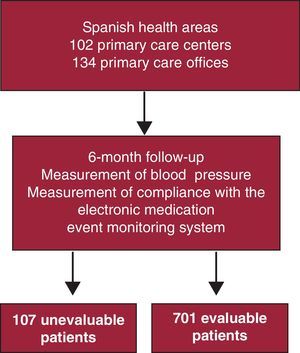
ResultsIn all, 701 patients (86.7%) completed the study and constitute the final sample. A total of 107 individuals were excluded, representing a loss of nearly 14% of the study population, for the following reasons: 7 because of travel or address changes, 3 because the antihypertensive therapy was discontinued, 46 did not use the MEMS, 42 because they did not keep their appointments with the investigator, and 10 due to malfunction of the MEMS. The overall mean age was 63.7 (11.1) years; there were 372 men (53.1%) and 329 women (46.9%). There were no significant differences in terms of age or sex (Figure 1). In all, 648 individuals complied with all the visits, although 701 were evaluable because they had all the pill counts in the MEMS until it was returned to the investigator.
Figure 1. General diagram of the study. Prospective, longitudinal study to evaluate compliance with antihypertensive therapy by means of the electronic medication event monitoring system in hypertensive patients at high vascular risk.
All the general characteristics of the sample are shown in Table 1. The SBP and DBP decreased significantly between the initial and final visits (P<.0001), 18.8mmHg and 9.8mmHg for the SBP and DBP, respectively (Table 2).
Table 1. Descriptive and Bivariate Analysis of Treatment Compliance.
| Variables | Total (n=701) | Compliers (n=514; 73.3%) | Noncompliers (n=187; 26.7%) | P |
| Sex | ||||
| Women | 329 (46.9) | 243 (47.3) | 86 (46) | ns |
| Men | 372 (53.1) | 271 (52.7) | 101 (54) | ns |
| Age, years | 63.7±11.1 | 63.6±11.1 | 63.9±11.2 | ns |
| Diagnosed diseases | 3.9±1.3 | 3.8±1.2 | 4.2±1.4 | ns |
| Number of drugs taken initially | 5.5±1.1 | 4.9±1.1 | 6.5±1.1 | <.001 |
| BMI | 30.3±2.9 | 30.2±2.8 | 30.5±3.1 | ns |
| WC, cm | 104.1±10.1 | 104.2±10 | 103.9±10.2 | ns |
| History of HT (years) | 5.7±3.1 | 5.6±3.2 | 5.9±3 | ns |
| Antihypertensive agent prescription | ns | |||
| Newly prescribed | 67 (9.6) | 51 (9.9) | 16 (8.6) | ns |
| Modification | 246 (35.1) | 180 (35) | 66 (35.3) | ns |
| Change | 388 (55.4) | 283 (55.1) | 105 (56.1) | ns |
| Patients prescribed additional antihypertensive agents during the course of the study | 145 (20.7) | 107 (20.8) | 38 (20.3) | ns |
| Age as a cardiovascular risk factor | 587 (83.7) | 430 (83.7) | 157 (84) | ns |
| Family history of early | ||||
| cardiovascular disease | 136 (19.4) | 102 (19.8) | 34 (18.2) | ns |
| Dyslipidemia | 475 (67.8) | 350 (68.1) | 125 (66.8) | ns |
| Diabetes mellitus | 385 (54.9) | 255 (49.6) | 130 (69.5) | <.001 |
| Smoking | 179 (25.5) | 134 (26.1) | 45 (24.1) | ns |
| Obesity | 329 (46.9) | 245 (47.7) | 84 (44.9) | ns |
| Left ventricular hypertrophy | 113 (16.1) | 83 (16.1) | 30 (16) | ns |
| Microalbuminuria | 116 (16.5) | 85 (16.5) | 31 (16.6) | ns |
| Retinopathy | 38 (5.4) | 28 (5.4) | 10 (5.3) | ns |
| Coronary artery disease | 245 (34.9) | 177 (34.4) | 68 (36.4) | ns |
| Peripheral vascular disease | 180 (25.7) | 136 (26.4) | 44 (23.5) | ns |
| Stroke | 108 (15.4) | 79 (15.4) | 29 (15.5) | ns |
BMI, body mass index; HT, hypertension; ns, not significant; WC, waist circumference.
Data expressed as no. (%) or mean±standard deviation.
Table 2. Overall Mean Systolic and Diastolic Blood Pressures at the Start of the Study and After 1, 3, and 6 Months.
| Initial | 1 month | 3 months | 6 months | P (initial vs final) | |
| SBP, mmHg | 149.5 (15) | 138.4 (16) | 134.7 (19.2) | 130.7 (24.9) | .0001 |
| DBP, mmHg | 86.3 (11.8) | 80.8 (10) | 78.7 (11.6) | 76.5 (14.8) | .0001 |
DBP, diastolic blood pressure; P (initial vs final), the differences between the initial and final blood pressure measurements were statistically significant; SBP, systolic blood pressure.
Results expressed as mean (standard deviation).
The mean percentage of doses taken was 87.9% (95%CI, 84.8%-91%); the mean percentage of days on which a dose of the antihypertensive drug was taken correctly, 73.4% (95%CI, 69.1%-77.7%), and the percentage of days on which the medication was taken at the correct time (from 7:00 to 9:00 AM), 63.17% (95%CI, 58.5%-67.8%). The therapeutic coverage was 82.4% (95%CI, 78.7%-86.1%).
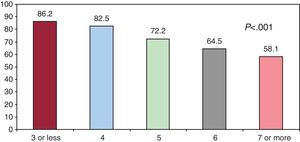
Overall, 73.3% (n=514) of the patients complied (95%CI, 69%-77.6%), 52.8% (n=370) complied with a once-daily dose (95%CI, 48%-57.6%), and 46.5% (n=326) complied in terms of timing (95%CI, 41.9%-51.1%). Table 3 shows the percentage of compliers classified according to the different PC. Figure 2 represents the percentage of compliers in terms of the number of pills prescribed.
Table 3. Distribution of the Patients According to the Percentage Compliance and Different Measures of Compliance.
| PC<80% | PC 80%-90% | PC>90% | |
| Overall compliance | 26.7% (n=187) | 8% (n=56) | 65.3% (n=458) |
| Compliance with once-daily dosing regimen | 47.2% (n=331) | 15.4% (n=108) | 37.4% (n=262) |
| Compliance with prescribed time frame | 53.5% (n=375) | 18.8% (n=132) | 27.7% (n=194) |
PC, percentage compliance.
Overall compliance: patients classified according to percentage compliance with the dosing regimen as a whole.
Compliance with once-daily dosing regimen: patients classified according to percentage of days on which they took their daily pill.
Compliance with prescribed time frame: patients classified according to the percentage of doses taken within the recommended time frame (from 7:00 to 9:00 AM).
Figure 2. Percentage of compliers according to the number of tablets prescribed.
The different patterns described are shown in Table 4.
Table 4. Percentages of Patients Grouped According to the Different Patterns of Compliance.
| Compliers | 73.3% (n=514) |
| Absolute complier | 9.2% (95%CI, ±2.6%) |
| Disguised complier | 20.5% (95%CI, ±3.9%) |
| Complier with sporadic noncompliance | 40.6% (95%CI, ±4.7%) |
| Overcomplier | 3% (95%CI, ±1.6%) |
| Noncompliers | 26.7% (n=187) |
| Absolute noncompliance | 4.1% (95%CI, ±1.9%) |
| Partial noncompliance | 20.3% (95%CI, ±3.9%) |
| Treatment suspension | 2.3% (95%CI, ±1.4%) |
| Total | 100% |
| Other patterns | |
| Expected noncompliance (≥80% or <80%) | 2.1% (95%CI, ±1.3%) |
| Drug holidays (≥80% or <80%) | 30% (95%CI, ±4.4%) |
| White-coat compliance (<80%) | 2.8% (95%CI, ±1.6%) |
| Noncompliance with dosing time frame | 47.2% (95%CI, ±4.8%) |
| Mixed noncompliance (≥80% or <80%) | 40.5% (95%CI, ±4.7%) |
95%CI, 95% confidence interval.
Bivariate analysis was carried out to assess the variables that can influence medication noncompliance (Table 1). Statistical significance was observed in the higher number of pills taken and the presence of diabetes (P<.001). To rule out the possibility that this be a confounding factor, we performed multivariate analysis with backward stepwise logistic regression. We introduced variables that, while not significant, could be relevant in clinical terms (age, sex, etc.). The model obtained was highly significant (P<.001) and the correct classification percentage was 79% (Table 5).
Table 5. Multivariate Analysis Taking Compliance/Noncompliance as a Dependent Variable.
| Variables | OR (95%CI) | P |
| Diabetes (no/yes) | 0.20 (0.12-0.32) | <.001 |
| Number of tablets | 0.52 (0.45-0.59) | <.001 |
95%CI, 95% confidence interval; OR, odds ratio.
Percentage classification, 79%.
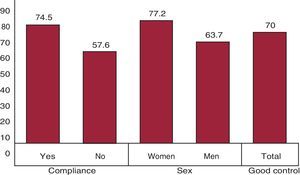
At one month, 50.1% of the patients had controlled HT (n=351); by month 3, control had been achieved in 62.9% (n=441), and at the end of the study, in 70% (n=491) (95%CI, 65.6%-74.4%). The increase in the percentage of controlled patients was significant (P=.0001). In the bivariate analysis, we observed significant differences, with a greater HT control among compliers versus noncompliers (P<.05) (Figure 3), and among women with respect to men (P<.05). In the multivariate analysis, the achievement of well-controlled HT was associated with compliance and sex (Table 6). The model was highly significant (P<.001) and was correctly predicted in 72% of the patients.
Figure 3. Bivariate analysis to assess the associations between good hypertension control and the statistically significant variables of the study (P<.05) expressed in percentages.
Table 6. Multivariate Analysis Taking Blood Pressure Control/Poor Blood Pressure Control as a Dependent Variable.
| Variables | OR (95%CI) | P |
| Compliance (yes/no) | 1.90 (1.47-2.52) | .011 |
| Sex (woman/man) | 1.69 (1.20-2.35) | .029 |
95%CI, 95% confidence interval; OR, odds ratio.
Percentage classification, 72%.
The absolute risk reduction in HT control was 19.9%; the RRR, 29%; the number of patients needed to treat, 5; and the RR, 0.71 (P<.001).
DiscussionThis study assessed compliance with antihypertensive therapy in patients at high vascular risk using the MEMS in a sample of 701 hypertensive patients taking an ACE inhibitor or an ARB, which constitutes the largest sample to date, at both the national and international level, of any study with this research objective. The clinical importance of using MEMS is highlighted by the great benefit achieved in early BP control, reducing the rate of cardiovascular events in high-risk hypertensive patients like those selected in the present study.7, 8
We observed a significant decreased in the BP levels; the incidence of HT control at the end of the study was 70%, and we consider noteworthy the fact that the percentage of controlled patients increased significantly in the follow-up visits and was higher among compliers than noncompliers. The magnitude of the clinical relevance of the intervention carried out in this investigation in HT control was important between the first month and the end of the study. It was necessary to treat 5 hypertensive patients with the intervention in order to avoid 1 case of poor HT control, and the protective effect is reflected by the RR lower than 1. This indicates that the intervention carried out by the investigators had a relevant clinical impact on HT control in hypertensive patients at high vascular risk.
Overall, 73.3% of the patients were compliers, but 52.8% complied with one pill a day, and 46.5% complied with the timing. As can be observed, the rate of noncompliance with taking all the pills was 26.7%. However, when the issue was whether the patients had taken a pill each day, the rate of noncompliance increased by 20%; this means that, for different reasons, many patients took two pills on some days and, on many other days, none at all (pattern of disguised compliance). Likewise, when the schedule of the medication is assessed, noncompliance with timing reaches 53.5%.
The prevalence of noncompliance in Spain ranges between 7.1% and 66.2%. In a review of all the studies on compliance published in Spain up to 2005, Márquez et al.6 observed that the weighted mean noncompliance was 32.53%. The rate of noncompliance obtained in our study (27%) reflects a better overall compliance with respect to the Spanish population with mild to moderate HT. In the studies in which compliance was measured using the MEMS, noncompliance was 17% in the EAPACUM study,1 13% in the CUMAMPA study,11 and 33.8% in the ECCON study in patients with mild to moderate HT.12 Outside of Spain, Van Onzenoort et al.,13 studying mild to moderate HT in the Dutch population, obtained an incidence of noncompliance of 9% in an intervention study involving home self-measurement of BP and 39% in another nonintervention study. In the United States, Lee et al.14 found a rate of noncompliance of 47%. In studies performed with ARB in German patients with mild HT treated with valsartan, Düsing et al.15 obtained a rate of noncompliers of 11.7% in the control group and 5% in the intervention group with informative measures. Mengden et al.16 observed that in patients with uncontrolled HT treated with candesartan/hydrochlorothiazide, the control of the disease and medication compliance improved. In the ETECUM study7 carried out in Spain, compliance was assessed by counting candesartan tablets, and a rate of noncompliance of 22% was observed in the control group and 15% in the telephone intervention group. Consequently, in general, noncompliance in patients at high vascular risk was more widespread than that observed in the literature. One of the factors may have been the greater number of pills taken by the patients compared to other studies involving patients with mild to moderate HT. In this study, we found a significant relationship between noncompliance, number of pills, and the presence of diabetes. The better compliance in the CUMPLE study was associated with fewer prescribed drugs and the absence of a diagnosis of diabetes. The incidence of HT control was higher among compliers and hypertensive women. In the multivariate analysis, it was observed that good HT control was associated with compliance and sex.
Knowledge of the prevalence of the different patterns of compliance and their influence on disease control could aid us in establishing a series of strategies to reduce medication noncompliance in our patients, since intervention in a patient with drug holidays will not be the same as that carried out in another with a pattern of white-coat compliance, basically if there was a relevant difference in its influence on the control of HT.
The percentage of absolute compliers—the patients who, during follow-up, took a pill every day—was found to be low (9.2%), showing that, to a greater or lesser extent, the remainder of the patients sometimes failed to comply, and this finding is highly relevant. The most common pattern among compliers was that of the patient who sporadically failed to comply, and that of noncompliers was that of the partial noncomplier. We stress the relevance of the pattern of drug holidays, which were recorded in 30% of the sample, and noncompliance with the time of medication, observed in 47.2%. Both patterns have a negative influence on HT control; the holidays, because the failure to take several doses of antihypertensive agents is followed by a substantial rise in BP on the third day, and noncompliance with the time frame will result in a reduced antihypertensive therapeutic coverage, with the ensuing higher daily antihypertensive pill burden.
The study could be relevant to the general population of patients with uncontrolled HT at high vascular risk treated in primary care, as the sizeable sample recruited by consecutive sampling is representative, with a relevant number of investigators from different primary care centers distributed throughout all the provinces of Spain. It meets the criteria recommended by Haynes et al.17 for compliance studies. Thus, the diagnosis of HT was correct, the method for measuring compliance—the MEMS—has been validated, the outcomes in terms of compliance and HT control were assessed, with follow-up of 80% of the sample in more than 50 individuals, and the magnitude of the clinical relevance of the intervention was calculated. The excluded patients (13%) may have introduced a bias, but the proportion was smaller than that previously calculated (18%), and our analysis demonstrated that their initial characteristics were similar to those of the evaluated patients. Likewise, knowledge of the study and the use of the MEMS and of the bulletins received periodically throughout the study by the physicians may have had the effect of increasing the intensity of the intervention on the part of the physician. However, these limitations are assumed in observational studies of health care efficiency in clinical practice and of clinical efficacy.18
The MEMS is the most widely recommended method for measuring compliance,19 despite the observation that compliance initially improves (Hawthorne effect), as does BP control,20, 21, 22 effects that diminish over time; the perceptions of the patients with respect to this method23 in terms of control and use24 are positive. The MEMS can aid in the detection of noncompliance as a cause of resistant25 and refractory26 HT and in the knowledge of the different patterns of noncompliance.
As a future line of research, we recommend studies designed with the aim of searching for strategies that maintain their efficacy over the long term, especially in these high-risk hypertensive patients who are taking combination drug therapy and have multiple diseases,27 because of the need to achieve early BP control to reduce cardiovascular morbidity and mortality.8, 9
ConclusionsIn poorly controlled hypertensive patients at high vascular risk, the intervention of the investigator carried out during follow-up achieves HT control in 2 out of 3 patients, and it is necessary to treat 5 patients to avoid 1 case of poor HT control. The rate of noncompliance is lower than that of the general Spanish hypertensive population, but higher than that observed in other studies involving the administration of specific ARB in hypertensive patients at lower vascular risk. Although the overall rate of compliance was 73.3%, 52.8% of the patients were once-daily compliers, and 46.5% complied with the prescribed time frame; the highest rate of noncompliance was recorded among those who had been prescribed more than 5 drugs, and there was a notably high incidence of the presentation of the patterns of noncompliance involving drug holidays and noncompliance with the time frame.
Conflicts of interestNone declared.
Acknowledgments
We wish to thank Laboratorios Boehringer Ingelheim for the grant awarded to finance the performance of the study.
☆ The Cumple-MEMS Study was the subject of an oral presentation in the VIII Jornadas Nacionales sobre Cumplimiento (II 8th Spanish Symposium on Compliance), held in February 2011 in Huelva, Spain.
Received 6 October 2011
Accepted 2 January 2012
Corresponding author: Puerto 7, 6.o D, 21003 Huelva, Spain. emarquezc@papps.org